Process hazards: Difference between revisions
No edit summary |
|||
| (18 intermediate revisions by 3 users not shown) | |||
| Line 3: | Line 3: | ||
Title: Process Hazards | Title: Process Hazards | ||
Authors: Anne Disabato, Tim Hanrahan, Brian Merkle | Authors: Anne Disabato, Tim Hanrahan, Brian Merkle (Winter 2014) | ||
Steward: David Chen, Fengqi You | |||
<br> | <br> | ||
| Line 16: | Line 17: | ||
The design and production of chemical processes is inherently hazardous, which is why process safety is of paramount importance to every company working in the chemical, fuels, and pharmaceuticals industry. While “process safety” focuses on the prevention of dangerous situation throughout the design, “process hazards” focuses on how to manage the unavoidable hazards in the final design. In the case of fires, explosions, or the release of toxic chemicals, proper safety hazard analysis will help minimize injuries and damage to the facility and environment. | The design and production of chemical processes is inherently hazardous, which is why process safety is of paramount importance to every company working in the chemical, fuels, and pharmaceuticals industry. While “process safety” focuses on the prevention of dangerous situation throughout the design, “process hazards” focuses on how to manage the unavoidable hazards in the final design. In the case of fires, explosions, or the release of toxic chemicals, proper safety hazard analysis will help minimize injuries and damage to the facility and environment. | ||
In addition to moral and ethical obligations to safety, law requires it and the costs (human, social, economic) of non-compliance can be catastrophic. | In addition to moral and ethical obligations to safety, law requires it and the costs (human, social, economic) of non-compliance can be catastrophic. Listed below are three major pieces of safety legislation (Towler and Sinnott, 2013): | ||
1. The Occupational Safety and Health Act (OSHA); 29 U.S.C. 651 et seq. (1970) | 1. The Occupational Safety and Health Act (OSHA); 29 U.S.C. 651 et seq. (1970) | ||
| Line 26: | Line 27: | ||
For safety organization and terminology, safe design tactics, and the economic cost of safety, please see [[Process safety]] | For safety organization and terminology, safe design tactics, and the economic cost of safety, please see [[Process safety]]. | ||
=Chemical Plant Hazards= | =Chemical Plant Hazards= | ||
| Line 34: | Line 35: | ||
===Toxicity=== | ===Toxicity=== | ||
Nearly every chemical plant is holding large quantities of various chemicals, which can be of serious concern for workers and local residents. Even chemicals with low toxicity can be deadly in the quantities used in manufacturing. Most exposure to high toxicity chemicals occurs from inhalation | Nearly every chemical plant is holding large quantities of various chemicals, which can be of serious concern for workers and local residents. Even chemicals with low toxicity can be deadly in the quantities used in manufacturing. Most exposure to high toxicity chemicals occurs from inhalation (Peters and Timmerhaus, 2003). Process design needs to consider the elimination or substitution of the most hazardous compounds, prevention of releases, containment, disposal, ventilation, and emergency procedures. | ||
The following are important toxicity definitions | The following are important toxicity definitions | ||
| Line 47: | Line 48: | ||
===Flammability=== | ===Flammability=== | ||
Flammability is the measurement of how easily a material will burn or ignite, resulting in a fire or combustion. A fire requires three things: fuel, oxidant, and source of ignition (or auto-ignition). Possible sources of ignition at a chemical facility should be assessed and eliminated; this include electrical equipment such as motors or actuators, open flames from furnaces, incinerators or flare stacks, and undefined sources such as matches, lighter or mobile phones | Flammability is the measurement of how easily a material will burn or ignite, resulting in a fire or combustion. A fire requires three things: fuel, oxidant, and source of ignition (or auto-ignition). Possible sources of ignition at a chemical facility should be assessed and eliminated; this include electrical equipment such as motors or actuators, open flames from furnaces, incinerators or flare stacks, and undefined sources such as matches, lighter or mobile phones (Biegler et al., 1997). | ||
Important flammability related properties must be measured: | Important flammability related properties must be measured: | ||
| Line 53: | Line 54: | ||
* Auto-ignition temperature- temperature at which the substance ignites in air spontaneously | * Auto-ignition temperature- temperature at which the substance ignites in air spontaneously | ||
* MSDS information | * MSDS information | ||
* Flammability limits- highest and lowest concentrations in air (NTP) at which a flame will propagate through the mixture | * Flammability limits- highest and lowest concentrations in air (NTP) at which a flame will propagate through the mixture (Peters and Timmerhaus, 2003) | ||
** LFL (lower flammable limit): mixture of fuel and air below this is too lean | ** LFL (lower flammable limit): mixture of fuel and air below this is too lean | ||
** UFL (Upper flammable limit): mixture of fuel and air below this will not burn | ** UFL (Upper flammable limit): mixture of fuel and air below this will not burn | ||
The LFL and UFL of mixtures can be calculated using Le Chatelier’s Equation | The LFL and UFL of mixtures can be calculated using Le Chatelier’s Equation (Seider et al., 2004): | ||
<math> FL_m = \frac {1} {\sum_{i=1}^C (y_i/FL_i)} </math> | <math> FL_m = \frac {1} {\sum_{i=1}^C (y_i/FL_i)} </math> | ||
where FL_i is the flammability limit of a specific component, and y_i is the concentration. While the LFL is relatively independent of pressure, the UFL changes at different pressures according to the following equation: | |||
where <math> FL_i </math> is the flammability limit of a specific component, and <math> y_i </math> is the concentration. While the LFL is relatively independent of pressure, the UFL changes at different pressures according to the following equation: | |||
<math> UFL_P = UFL + 20.6[log(P)+1] </math> | <math> UFL_P = UFL + 20.6[log(P)+1] </math> | ||
| Line 69: | Line 72: | ||
Fire protection is best accomplished by containing flammable materials. Other tactics include: | Fire protection is best accomplished by containing flammable materials. Other tactics include: | ||
* Inerting- an inert gas is added to reduce the oxygen concentration below the minimum oxygen concentration | * Inerting- an inert gas is added to reduce the oxygen concentration below the minimum oxygen concentration (MOC) at which explosions can occur | ||
* Reducing static electricity- by installing ground devices or using antistatic additive to increase conductivity | * Reducing static electricity- by installing ground devices or using antistatic additive to increase conductivity | ||
* Explosion-proof equipment- designed to absorb shock after explosion and prevent the combustion from spreading. | * Explosion-proof equipment- designed to absorb shock after explosion and prevent the combustion from spreading. | ||
| Line 93: | Line 96: | ||
===Fires and Explosions=== | ===Fires and Explosions=== | ||
Chemical plant fires can quickly damage control systems and equipment, causing overpressure, loss of containment, and explosions. In addition to protecting expensive equipment, the safety and lives of workers, local residents, and the environment are put at risk if a fire starts. It is important to follow fire protection guidelines (NFPA 30, API RP 2001, API Publ 2218) and legal requirements set by OSHA (29 CFR 1910 L) | Chemical plant fires can quickly damage control systems and equipment, causing overpressure, loss of containment, and explosions. In addition to protecting expensive equipment, the safety and lives of workers, local residents, and the environment are put at risk if a fire starts. It is important to follow fire protection guidelines (NFPA 30, API RP 2001, API Publ 2218) and legal requirements set by OSHA (29 CFR 1910 L) (Towler and Sinnott, 2013). | ||
As mentioned in the “Flammability” section, the use of electrical equipment in chemical plants can ignite a fire. Electrical equipment use is regulated by law through OSHA standard 29 CFR 1910.307 and industry design codes, National Electrical Code NFPA 70 and NFPA standards 496,497, API RP 500, 505 | As mentioned in the “Flammability” section, the use of electrical equipment in chemical plants can ignite a fire. Electrical equipment use is regulated by law through OSHA standard 29 CFR 1910.307 and industry design codes, National Electrical Code NFPA 70 and NFPA standards 496,497, API RP 500, 505 (Towler and Sinnott, 2013). These codes define equipment and installation regulations, and specific precautions that must be taken in risky areas. | ||
An explosion is a worst-case result of a fire, defined as the sudden, catastrophic release of energy causing a pressure wave. The following are explosion related definitions: | An explosion is a worst-case result of a fire, defined as the sudden, catastrophic release of energy causing a pressure wave. The following are explosion related definitions: | ||
| Line 102: | Line 105: | ||
* Expansion factor- measure of the increase in volume resulting from combustion: | * Expansion factor- measure of the increase in volume resulting from combustion: | ||
<math> E = \frac{ | <math> E = \frac{\rho_R} {\rho_P} </math> | ||
where the maximum value of E is for adiabatic combustion | where <math> \rho_R </math> is the molar density of the reagents and <math> \rho_P </math> is the molar density of the products, and the maximum value of E is for adiabatic combustion (Towler and Sinnott, 2013). | ||
* Flame speed- the rate of propagation of a flame front through a flammable mixture, with respect to a fixed observer | * Flame speed- the rate of propagation of a flame front through a flammable mixture, with respect to a fixed observer | ||
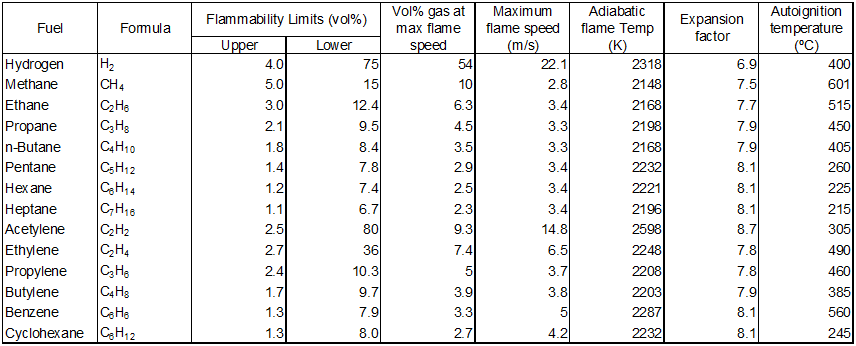
Explosivity properties can be found in textbooks such as An Introduction to Fire Dynamics by | Explosivity properties can be found in textbooks such as An Introduction to Fire Dynamics by Dugdale (1985), as shown below. | ||
Figure 1 | [[File:Hazards 1.PNG|center|800px|frame|Figure 1. Explosivity Properties (Dugdale, 1985).]] | ||
===Loss of Containment=== | ===Loss of Containment=== | ||
Loss of containment can occur due to pressure relief, operator error, poor maintenance procedures, such as failure to drain and purge properly, or leaks from degraded equipment. | Loss of containment can occur due to pressure relief, operator error, poor maintenance procedures, such as failure to drain and purge properly, or leaks from degraded equipment. | ||
Containment is one of the six principles of “Inherently Safe Design,” laid out in the wiki page for [[Process safety]] | Containment is one of the six principles of “Inherently Safe Design,” laid out in the wiki page for [[Process safety]] (Turton et al., 2003). If hazardous materials cannot be eliminated, they should at least be stored in vessels with mechanical integrity beyond any reasonably expected temperature or pressure excursion. This is an old but effective strategy to avoid leaks. However, it is not as inherently safe as substitution, intensification, or attenuation. | ||
===Noise=== | ===Noise=== | ||
| Line 125: | Line 126: | ||
Sound is measured in decibels, defined by the following equation: | Sound is measured in decibels, defined by the following equation: | ||
<math> | <math> SL = 20 \log_{10} \frac{P_S} {0.00002} </math> | ||
where <math> P_S </math> is the root mean squared sound pressure and the result (SL) is a sound level in dB. It is advised to wear ear protection in areas over 80 dB, as permanent damage can be caused by noise over 85 dB (Towler and Sinnott, 2013). | |||
=Process Hazard Analysis Tools= | =Process Hazard Analysis Tools= | ||
==Exposure Evaluation== | ==Exposure Evaluation== | ||
At each stage of the process, every chemical, intermediate, and catalyst must be inventoried and analyzed. Risk can then be prioritized through a combination of chemical toxicity and exposure source (valve, pump, etc.). Exposure is highly variable, and should be measure over relatively large time period, longer than chemicals with long half-life. Finally, compliance with OSHA requires designer to make calculations of concentrations and exposure time of plant personnel during normal operation | At each stage of the process, every chemical, intermediate, and catalyst must be inventoried and analyzed. Risk can then be prioritized through a combination of chemical toxicity and exposure source (valve, pump, etc.). Exposure is highly variable, and should be measure over relatively large time period, longer than chemicals with long half-life. Finally, compliance with OSHA requires designer to make calculations of concentrations and exposure time of plant personnel during normal operation (Peters and Timmerhaus, 2003). | ||
==MSDS== | ==MSDS== | ||
| Line 139: | Line 140: | ||
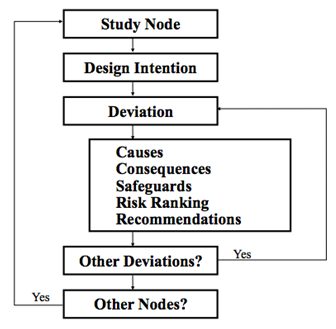
==Hazard and Operability Study (HAZOP)== | ==Hazard and Operability Study (HAZOP)== | ||
A HAZOP is a structured and systematic examination of a planned or existing process or operation in order to identify and evaluate problems that may represent risk to personnel or equipment, or prevent efficient operation | A HAZOP is a structured and systematic examination of a planned or existing process or operation in order to identify and evaluate problems that may represent risk to personnel or equipment, or prevent efficient operation (Peters and Timmerhaus, 2003). HAZOP analysis is required by the following government regulations: | ||
* USA OSHA 29 CFR 1910.119 [Process Safety Management Standard] | * USA OSHA 29 CFR 1910.119 [Process Safety Management Standard] | ||
* USA EPA 40 CFR 68 [Risk Management Program] | * USA EPA 40 CFR 68 [Risk Management Program] | ||
* Regulations under the European Seveso Directive | * Regulations under the European Seveso Directive | ||
[[File:Hazards 2.PNG|center|300px | [[File:Hazards 2.PNG|center|300px|frame|Figure 2. Flowsheet depicting general guidelines for how to do a HAZOP.]] | ||
Figure 2 | |||
==Fault-Tree Analysis (FTA)== | ==Fault-Tree Analysis (FTA)== | ||
| Line 154: | Line 154: | ||
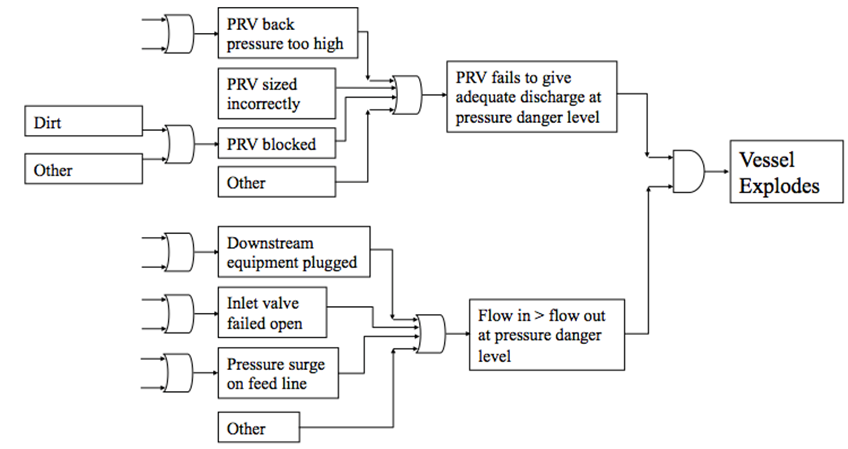
For example, imagine that you are tasked with performing a FTA on a pressure vessel. First, all of the possible causes of hazards associated with the pressure vessel are explored. These hazard sources are then analyzed to see how they dangerously affect the system, and then to see the final result if left unchecked. An example FTA is shown in Figure 3 below. | For example, imagine that you are tasked with performing a FTA on a pressure vessel. First, all of the possible causes of hazards associated with the pressure vessel are explored. These hazard sources are then analyzed to see how they dangerously affect the system, and then to see the final result if left unchecked. An example FTA is shown in Figure 3 below. | ||
[[File:Hazards 3.PNG|center|500px | [[File:Hazards 3.PNG|center|500px|frame|Figure 3. Example FTA for Pressure Vessel (Cole, 2013).]] | ||
Figure 3 | |||
==Failure Mode-and-Effect Analysis (FMEA)== | ==Failure Mode-and-Effect Analysis (FMEA)== | ||
FMEA is an early stage approach to identifying critical technical risks using a semi-quantitative procedure. See the [[Process safety]] wiki page for more information. | FMEA is an early stage approach to identifying critical technical risks using a semi-quantitative procedure. See the [[Process safety]] wiki page for more information. | ||
=Conclusions= | |||
In conclusion, the hazards described above are only a few of the hazards that can be present in chemical processes, and it is important that steps are being taken to mitigate these hazards at every stage of the design process, be it through FMEA, FTA, HAZOP, or other safety analyses. Proper safety hazard analysis will help minimize injuries and damage to the facility and environment, thus saving a company time and money, all while protecting the health of its employees. | |||
=References= | =References= | ||
Biegler LT, Grossmann IE, Westerberg AW. Systematic Methods of Chemical Process Design. Upper Saddle River: Prentice Hall; 1997. | |||
Cole JL. Chemical Engineering 351 Process Economics, Design, and Evaluation [Lecture Slides]. Evanston: Northwestern University; 2013. | |||
Dugdale D. An Introduction to Fire Dynamics. New York: Wiley; 1985. | |||
Peters MS, Timmerhaus KD. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw Hill; 2003. | |||
Seider WD, Seader JD, Lewin DR. Process Design Principles: Synthesis, Analysis, and Evaluation. 3rd ed. New York: Wiley; 2004. | |||
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013. | |||
Turton R, Bailie RC, Whiting WB, Shaewitz JA, Bhattacharyya D. Analysis, Synthesis, and Design of Chemical Processes. 4th ed. Upper Saddle River: Prentice-Hall; 2012. | |||
Latest revision as of 04:16, 2 March 2015
Title: Process Hazards
Authors: Anne Disabato, Tim Hanrahan, Brian Merkle (Winter 2014)
Steward: David Chen, Fengqi You
Introduction
The design and production of chemical processes is inherently hazardous, which is why process safety is of paramount importance to every company working in the chemical, fuels, and pharmaceuticals industry. While “process safety” focuses on the prevention of dangerous situation throughout the design, “process hazards” focuses on how to manage the unavoidable hazards in the final design. In the case of fires, explosions, or the release of toxic chemicals, proper safety hazard analysis will help minimize injuries and damage to the facility and environment.
In addition to moral and ethical obligations to safety, law requires it and the costs (human, social, economic) of non-compliance can be catastrophic. Listed below are three major pieces of safety legislation (Towler and Sinnott, 2013):
1. The Occupational Safety and Health Act (OSHA); 29 U.S.C. 651 et seq. (1970)
- Employers must provide a place of employment free from recognized hazards to safety and health, such as exposure to toxic chemicals, excessive noise levels, mechanical dangers, heat or cold stress, or unsanitary conditions.
2. The Emergency Planning & Community Right-To-Know Act (EPCRA); 42 U.S.C. 11011 et seq. (1986)
- To help local communities protect public health, safety, and the environment from chemical hazards.
3. The Toxic Substances Control Act (TSCA); 15 U.S.C. s/s 2601 et seq. (1976)
- Allows EPA to track industrial chemicals and ban their manufacture or import
For safety organization and terminology, safe design tactics, and the economic cost of safety, please see Process safety.
Chemical Plant Hazards
The complex nature of chemical plants increases the number of hazards associated with operation and facility maintenance. Understanding the scope of (1) material and (2) process hazards is essential to safe design and operation. Below are examples of chemical plant hazards.
Material Hazards
Toxicity
Nearly every chemical plant is holding large quantities of various chemicals, which can be of serious concern for workers and local residents. Even chemicals with low toxicity can be deadly in the quantities used in manufacturing. Most exposure to high toxicity chemicals occurs from inhalation (Peters and Timmerhaus, 2003). Process design needs to consider the elimination or substitution of the most hazardous compounds, prevention of releases, containment, disposal, ventilation, and emergency procedures.
The following are important toxicity definitions
- Acute Effects- Symptoms that develop rapidly, usually as a result of short-term exposure. These effects can be a result of oral, dermal, gas, vapor, dust, or mist inhalation.
- Chronic Effects: Symptoms that develop over a long period of time, often as a result of long-term exposure. Example: Cancer
- LD50- Lethal dose at which 50% of test animals are killed. Indicates acute effects only, expressed in mg/kg body mass
- Threshold Limit Value (TLV) or Permissible Exposure Limit (PEL)- Concentration the average worker can safely be exposed to for 40 hr/week
- PEL published by OSHA : http://www.osha.gov/SLTC/healthguidelines/
- TLV published by American Conference of Government Industrial Hygienists. http://www.acgih.org/home.htm
Toxic Substance Control Act or TSCA (15 U.S.C. s/s 2601 et seq., 1976) is USEPA’s version of the Food and Drug Act. The TSCA allows EPA to regulate the 75,000 chemical substances used in industry (including confidential materials). Additionally, it requires extensive review before approval is given by USEPA to manufacture, import and sell a new chemical in the USA. Under TSCA, USEPA can ban or restrict the import, manufacture and use of any chemical, and anyone has the right and obligation to report information about new or alleged health/environmental effects caused by a chemical.
Flammability
Flammability is the measurement of how easily a material will burn or ignite, resulting in a fire or combustion. A fire requires three things: fuel, oxidant, and source of ignition (or auto-ignition). Possible sources of ignition at a chemical facility should be assessed and eliminated; this include electrical equipment such as motors or actuators, open flames from furnaces, incinerators or flare stacks, and undefined sources such as matches, lighter or mobile phones (Biegler et al., 1997).
Important flammability related properties must be measured:
- Flash Point – function of vapor pressure; lowest temperature at which the material will ignite from an open flame
- Auto-ignition temperature- temperature at which the substance ignites in air spontaneously
- MSDS information
- Flammability limits- highest and lowest concentrations in air (NTP) at which a flame will propagate through the mixture (Peters and Timmerhaus, 2003)
- LFL (lower flammable limit): mixture of fuel and air below this is too lean
- UFL (Upper flammable limit): mixture of fuel and air below this will not burn
The LFL and UFL of mixtures can be calculated using Le Chatelier’s Equation (Seider et al., 2004):
where is the flammability limit of a specific component, and is the concentration. While the LFL is relatively independent of pressure, the UFL changes at different pressures according to the following equation:
where P is in MPa and UFL is the upper flammability limit at 1 atmosphere.
Fire protection is best accomplished by containing flammable materials. Other tactics include:
- Inerting- an inert gas is added to reduce the oxygen concentration below the minimum oxygen concentration (MOC) at which explosions can occur
- Reducing static electricity- by installing ground devices or using antistatic additive to increase conductivity
- Explosion-proof equipment- designed to absorb shock after explosion and prevent the combustion from spreading.
- Flame arrestors- specified on vent lines of equipment that contains flammable materials to prevent a flame from propagating back from the vent
- Sprinkler systems
Incompatibility
When certain hazardous chemicals are stored or mixed together, violent reactions may occur because the chemicals are incompatible. Combination of interest include:
- Acids and Bases
- Acids and Metals
- Fuels and Oxidants
- Free radical initiators and Epoxides, Peroxides, or Unsaturates.
Chemical incompatibility can lead to runaway reactions, and material incompatibility can lead to corrosion of vessels, internals, and instruments as well as the softening of gaskets, seals, and linings.
Material Hazards Conclusions
Material hazards account for a wide variety of incidents. Six factors should be considered in design for material hazards: (1) substitution, (2) containment, (3) prevention of releases, (4) ventilation, (5) disposal, and (6) provision of emergency equipment. Consulting the Material Safety Data Sheets (MSDS) is also essential in accounting for hazards. Please see the MSDS section under “Process Hazard Analysis Tools.”
Process Hazards
Overpressure
Overpressure occurs when mass, moles or energy accumulates in a contained volume (or space with restricted outflow), and can be extremely dangerous. The rise in pressure is determined by the rate of accumulation. Process controls are one tool used to control process pressures, but in the case of overpressure, they may not be able to response quickly enough. If pressure is not released by a pressure safety value, a vessel could rupture or explode resulting in the loss of containment. Please see Process hydraulics and Pressure Vessels for help with creating an appropriate design. Pressure relief values and rupture disks should be installed on all pressure vessels.
Fires and Explosions
Chemical plant fires can quickly damage control systems and equipment, causing overpressure, loss of containment, and explosions. In addition to protecting expensive equipment, the safety and lives of workers, local residents, and the environment are put at risk if a fire starts. It is important to follow fire protection guidelines (NFPA 30, API RP 2001, API Publ 2218) and legal requirements set by OSHA (29 CFR 1910 L) (Towler and Sinnott, 2013).
As mentioned in the “Flammability” section, the use of electrical equipment in chemical plants can ignite a fire. Electrical equipment use is regulated by law through OSHA standard 29 CFR 1910.307 and industry design codes, National Electrical Code NFPA 70 and NFPA standards 496,497, API RP 500, 505 (Towler and Sinnott, 2013). These codes define equipment and installation regulations, and specific precautions that must be taken in risky areas.
An explosion is a worst-case result of a fire, defined as the sudden, catastrophic release of energy causing a pressure wave. The following are explosion related definitions:
- Deflagration- an exposition where combustion zone propagates at subsonic flame speed, usually < 30 m/s, and pressure wave < 10 bar. Deflagration can turn into detonation when propagating along a pipe.
- Detonation- an exposition where combustion zone propagates at supersonic velocity, 2000 – 3000 m/s, and pressure wave 20 bar. The principal heating mechanism is shock compression, and requires confinement of a high-intensity source
- Expansion factor- measure of the increase in volume resulting from combustion:
where is the molar density of the reagents and is the molar density of the products, and the maximum value of E is for adiabatic combustion (Towler and Sinnott, 2013).
- Flame speed- the rate of propagation of a flame front through a flammable mixture, with respect to a fixed observer
Explosivity properties can be found in textbooks such as An Introduction to Fire Dynamics by Dugdale (1985), as shown below.
Loss of Containment
Loss of containment can occur due to pressure relief, operator error, poor maintenance procedures, such as failure to drain and purge properly, or leaks from degraded equipment.
Containment is one of the six principles of “Inherently Safe Design,” laid out in the wiki page for Process safety (Turton et al., 2003). If hazardous materials cannot be eliminated, they should at least be stored in vessels with mechanical integrity beyond any reasonably expected temperature or pressure excursion. This is an old but effective strategy to avoid leaks. However, it is not as inherently safe as substitution, intensification, or attenuation.
Noise
Noise may not seem like a process hazard when compared to explosions, but can cause permanent damage to hearing. Compressors, turbines, motors, and solids handling can be very noisy, both within the plant and in the surrounding neighborhood.
Sound is measured in decibels, defined by the following equation:
where is the root mean squared sound pressure and the result (SL) is a sound level in dB. It is advised to wear ear protection in areas over 80 dB, as permanent damage can be caused by noise over 85 dB (Towler and Sinnott, 2013).
Process Hazard Analysis Tools
Exposure Evaluation
At each stage of the process, every chemical, intermediate, and catalyst must be inventoried and analyzed. Risk can then be prioritized through a combination of chemical toxicity and exposure source (valve, pump, etc.). Exposure is highly variable, and should be measure over relatively large time period, longer than chemicals with long half-life. Finally, compliance with OSHA requires designer to make calculations of concentrations and exposure time of plant personnel during normal operation (Peters and Timmerhaus, 2003).
MSDS
Material Safety and Data Sheet - Every chemical has an MSDS which contains all the information regarding safe handling and how to deal with spills or other accidents involving the substance. Relevant information includes how to identify the substance, hazard information, and how to handle spills, fires, and exposure, among other things.
In process design, it is important to collect the MSDS of all components used in the process at as early a stage as possible in order to identify potential hazards that may arise later.
Hazard and Operability Study (HAZOP)
A HAZOP is a structured and systematic examination of a planned or existing process or operation in order to identify and evaluate problems that may represent risk to personnel or equipment, or prevent efficient operation (Peters and Timmerhaus, 2003). HAZOP analysis is required by the following government regulations:
- USA OSHA 29 CFR 1910.119 [Process Safety Management Standard]
- USA EPA 40 CFR 68 [Risk Management Program]
- Regulations under the European Seveso Directive
Fault-Tree Analysis (FTA)
Along with a reliability analysis, a fault tree analysis is a quantitative method to estimate the probability of an event based on known probabilities such as the probability of failure of an instrument.
In a FTA, designers form an event diagram that describes the routes by which a hazard may occur; Boolean algebra and component probabilities are used to determine overall probability of the hazard. The design can then be modified until desired hazard rate is achieved.
For example, imagine that you are tasked with performing a FTA on a pressure vessel. First, all of the possible causes of hazards associated with the pressure vessel are explored. These hazard sources are then analyzed to see how they dangerously affect the system, and then to see the final result if left unchecked. An example FTA is shown in Figure 3 below.
Failure Mode-and-Effect Analysis (FMEA)
FMEA is an early stage approach to identifying critical technical risks using a semi-quantitative procedure. See the Process safety wiki page for more information.
Conclusions
In conclusion, the hazards described above are only a few of the hazards that can be present in chemical processes, and it is important that steps are being taken to mitigate these hazards at every stage of the design process, be it through FMEA, FTA, HAZOP, or other safety analyses. Proper safety hazard analysis will help minimize injuries and damage to the facility and environment, thus saving a company time and money, all while protecting the health of its employees.
References
Biegler LT, Grossmann IE, Westerberg AW. Systematic Methods of Chemical Process Design. Upper Saddle River: Prentice Hall; 1997.
Cole JL. Chemical Engineering 351 Process Economics, Design, and Evaluation [Lecture Slides]. Evanston: Northwestern University; 2013.
Dugdale D. An Introduction to Fire Dynamics. New York: Wiley; 1985.
Peters MS, Timmerhaus KD. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw Hill; 2003.
Seider WD, Seader JD, Lewin DR. Process Design Principles: Synthesis, Analysis, and Evaluation. 3rd ed. New York: Wiley; 2004.
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.
Turton R, Bailie RC, Whiting WB, Shaewitz JA, Bhattacharyya D. Analysis, Synthesis, and Design of Chemical Processes. 4th ed. Upper Saddle River: Prentice-Hall; 2012.



![{\displaystyle UFL_{P}=UFL+20.6[log(P)+1]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/dd56f14abb3947cc11b6b004ed7fd3e7580d5c09)