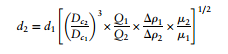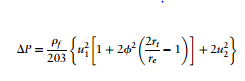Separation processes: Difference between revisions
| (176 intermediate revisions by 7 users not shown) | |||
| Line 2: | Line 2: | ||
<br> | <br> | ||
Authors: Nick Pinkerton,<sup> [2014] </sup> Karen Schmidt,<sup> [2014] </sup> James Xamplas,<sup> [2014] </sup> Emm Fulk,<sup> [2015] </sup> and Erik Zuehlke <sup> [2015] </sup> | Authors: Nick Pinkerton,<sup> [2014] </sup> Karen Schmidt,<sup> [2014] </sup> James Xamplas,<sup> [2014] </sup> Emm Fulk,<sup> [2015] </sup> and Erik Zuehlke, <sup> [2015] </sup> John Dombrowski <sup> [2016] </sup>, Brett Sleyster <sup> [2016] </sup>, Robert Cignoni <sup> [2016] </sup>, and Osman Jamil <sup> [2016] </sup> | ||
Stewards: David Chen, Jian Gong, and Fengqi You | Stewards: David Chen, Jian Gong, and Fengqi You | ||
| Line 14: | Line 14: | ||
Essentially all chemical processes require the presence of a separation stage. Most chemical plants comprise of a reactor surrounded by many separators. Separators have a countless number of jobs inside of a chemical plant. A separator can process raw materials prior to the reaction, remove incondensable gases, remove undesired side products, purify a product stream, recycle materials back into the process, and many other jobs that are essential to the process. | Essentially all chemical processes require the presence of a separation stage. Most chemical plants comprise of a reactor surrounded by many separators. Separators have a countless number of jobs inside of a chemical plant. A separator can process raw materials prior to the reaction, remove incondensable gases, remove undesired side products, purify a product stream, recycle materials back into the process, and many other jobs that are essential to the process. | ||
Chemical engineers must understand the science of separation and the variety of ways that separation can take place. There are many ways to perform a separation some of these including: distillation, absorption, stripping, and extraction. The science of separation revolves around the presence of two phases that are in contact and equilibrium | Chemical engineers must understand the science of separation and the variety of ways that separation can take place. There are many ways to perform a separation some of these including: distillation, absorption, stripping, and extraction. The science of separation revolves around the presence of two phases that are in contact and equilibrium (Wankat, 2012). | ||
[[File:Sepmeth.JPG|frame|Figure 1. Separation methods by property]] | [[File:Sepmeth.JPG|frame|Figure 1. Separation methods by property]] | ||
| Line 38: | Line 38: | ||
===Column Distillation=== | ===Column Distillation=== | ||
Distillation columns are the most widely used separation technique used in the chemical industry, accounting for approximately 90% of all separations | Distillation columns are the most widely used separation technique used in the chemical industry, accounting for approximately 90% of all separations (Wankat, 2012). Distillations in columns consist of multiple trays that each act at their own equilibrium conditions. Large columns are able to perform complete separations of binary mixtures as well as more complex multi-component mixtures. | ||
[[File:column.jpg|250px|center|]] | [[File:column.jpg|250px|center|]] | ||
| Line 47: | Line 47: | ||
[[File:sieve.jpg|200px|center|]] | [[File:sieve.jpg|200px|center|]] | ||
====Sieve Tray Design Procedure==== | |||
The design of these plates is done through a trial-and-error process. Most commercial process simulations (such as HYSYS) have default tray designs, and automatically specify dimensions. However, these dimensions selected or calculated by the simulations may not give the best performance for your system, so it is valuable to understand how to design the sieve trays and how specific parameters may affect performance. Hand calculations using the following methods can be used to guide the simulation programs to better design. This section will use sample data to work through an example of the process. The following is a general list of steps for designing a sieve plate: | |||
=====1. Calculate the maximum and minimum vapor and liquid flow rates for the turndown ratio required.===== | |||
This data can be collected from a McCabe-Thiele diagram and/or from process simulation data. | |||
<u>Data from McCabe Thiele diagram, for example</u>: <br /> | |||
Number of stages = 10 <br /> | |||
Slope of top operating line = 0.185 <br /> | |||
Slope of bottom operating line = 1.43 <br /> | |||
Top composition = 98.8 mol% acetone <br /> | |||
Bottom composition = 4 mol% acetone (a) <br /> | |||
Minimum reflux ratio = 0.31 <br /> | |||
<u>Collect data rom mass balances:</u><br /> | |||
MW of feed = (0.6 kmol a/kmol)*(58 kg/kmol a) + (0.4 kmol acetic acid/kmol)*(60 kg/kmol aa) = 59 kg/kmol <br /> | |||
Feed rate = 100 kmol/h <br /> | |||
Using material balances... D = 57.3 kmol/h ; B = 42.7 kmol/h<br /> | |||
Vapor rate, V = D(1+R) = 57.3 kmol/h (1+.31) = 75.1 kmol/h <br /> | |||
Below feed line, slope of BOL = 1.43 = L'<sub>m</sub>/V'<sub>m</sub> (liquid rate/vapor rate) <br /> | |||
-- Also, V'<sub>m</sub> = L'<sub>m</sub> - B (mass balance on bottom of column)<br /> | |||
-- V'<sub>m</sub>= 99.3 kmol/h and L'<sub>m</sub> = 142.0 kmol/h | |||
=====2. Collect or estimate the system physical properties.===== | |||
Here it is important to know information about both the top and bottom of the column. Useful information includes temperature, pressure, column pressure drop (a common assumption is 100 mmH2O per plate), densities, molecular weights, surface tensions, and number of stages (which can be estimated from the McCabe-Thiele diagram). <br /> | |||
<br /> | |||
<u>Conditions at the top of the column</u>:<br /> | |||
T = 59<sup>o</sup>C<br /> | |||
P = 760 mmHg = 101,333 N/m<sup>2</sup> (atmospheric pressure)<br /> | |||
<dfn>Estimate the properties of the top as those of the vapor (acetone here), which is typically the only thing present in binary distillation. </dfn> <br /> | |||
Surface tension = 18.9 x 10<sup>-3</sup> N/m<br /> | |||
<math>\rho_v</math> = 2.55 kg/m<sup>3</sup><br /> | |||
<math>\rho_L</math> = 790 kg/m<sup>3</sup><br /> | |||
MW = 58 kg/kmol<br /> | |||
<br /> | |||
<u>Conditions at bottom of the column</u>:<br /> | |||
T = 104<sup>o</sup>C | |||
Pressure -- There is a pressure drop in the column, from the bottoms to the top. A common assumption is a pressure drop per plate of 100 mmH<sub>2</sub>O <br /> | |||
Change in P = (9.81 x 10<sup>3</sup> Pa/mmH<sub>2</sub>O)(100 mmH<sub>2</sub>O)(1000 kgH<sub>2</sub>O/m<sup>3</sup>)(16 plates) = 15,696 N/m<sup>2</sup> <br /> | |||
P<sub>bottoms</sub> = P<sub>atm</sub> + P<sub>gauge</sub> = 101,333 N/m<sup>2</sup> + 15,696 N/m<sup>2</sup> = 117,030 N/m<sup>2</sup> <br /> | |||
<br /> | |||
<dfn>Estimate the properties of the bottoms as those of the heavy component (acetic acid here)</dfn>:<br /> | |||
Surface tension = 13.9 x 10<sup>-3</sup> N/m<br /> | |||
<math>\rho_v</math> = 2.68 kg/m<sup>3</sup><br /> | |||
<math>\rho_L</math> = 1049 kg/m<sup>3</sup><br /> | |||
MW = 60 kg/kmol<br /> | |||
=====3. Select a Trial Plate Spacing===== | |||
The plate spacing will depend on the column diameter and operating conditions. Plate spacings from 0.15 m to 1.0 m are typically used. The smaller the diameter, the smaller the spacing. Small columns will use close spacing. Columns with diameters above 1.0 m, plate spacings of 0.3 m to 0.6 m are normally used. A good initial estimate is 0.5 m. | |||
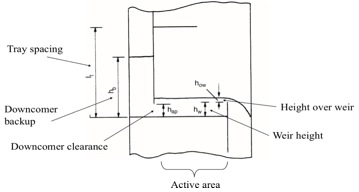
[[File:trayspacing.jpg|400px|center|frame|Figure 3: Tray diagram.]] | |||
=====4. Estimate the column diameter, based on flooding considerations.===== | |||
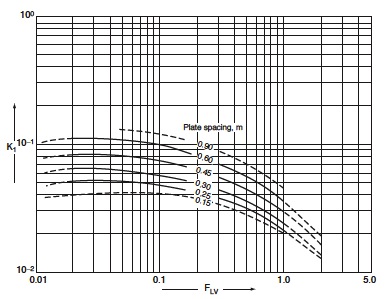
Vapor and liquid flow rates will vary along the column, so plate design needs to be considered both above and below the feed. Using plate spacing and F<sub>LV</sub>, you can obtain the value of K from Figure 4.<br /> | |||
<math>F_{\text{LV}} = (slope_{\text{operating line}})*\sqrt{\frac{\rho_v}{\rho_L}}</math><br /> | |||
<math>F_{\text{LV(bottom)}} = 0.072</math><br /> | |||
<math>F_{\text{LV(top)}} = 0.011</math><br /> | |||
Thus, K<sub>1_bottom</sub> = 0.90 and K<sub>1_top</sub> = 0.85 <br /> | |||
<dfn>Next, correct those values of K<sub>1</sub> for surface tensions:</dfn> | |||
<math>K_1' = K_1*(\frac{\sigma}{\sigma_{\text{water}}})^{\text{0.2}}</math><br /> | |||
<math>\sigma = {\text{surface tension}} </math><br /> | |||
<dfn>Next, calculate u<sub>f</sub>, the vapor velocity through the net area at flooding:</dfn><br /> | |||
<math>u_f = K_1\sqrt{\frac{\rho_L - \rho_V}{\rho_V}}</math> | |||
[[File:Fig1129.jpg|100px|center|frame|Figure 4: Flooding velocity, sieve plates.]] | |||
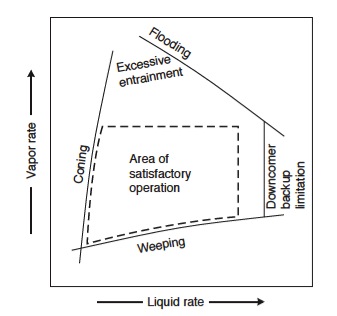
There is a range of vapor and liquid flow rates in which the column needs to be operated. Too low or too high of rates can result in various inefficiencies in the column operation, as shown in Figure 5. For example, if the vapor rate is too high, flooding will occur. However, it is not safe to operate on the flooding line. Instead, columns are typically designed for 80% of flooding at the maximum flow rate. <br /> | |||
Obtain a new velocity with this 80%, and use the velocity to calculate a a maximum volumetric flowrate. Using this and the velocity, we can calculate a net area necessary for vapor flow through the plate. Also need to assume a downcomer area. Now a column corss sectional area can be calculated. | |||
[[File:Fig1128.jpg|400px|center|frame|Figure 5: Sieve plate performance diagram.]] | |||
=====5. Decide the liquid flow arrangement.===== | |||
Common flow arrangements are single pass (cross flow), double pass, and reverse flow. Using conditions at the bottom of the column, calculate the max volumetric flow rate. Use this flow rate and the column diameter to determine the preferred flow arrangement from the chart below. | |||
[[File:Fig1130.jpg|200px|center|frame|Figure 6: Selection of liquid-flow arrangement]] | |||
=====6. Make a trial plate layout: downcomer area, active area, hole area, hole size, weir height.===== | |||
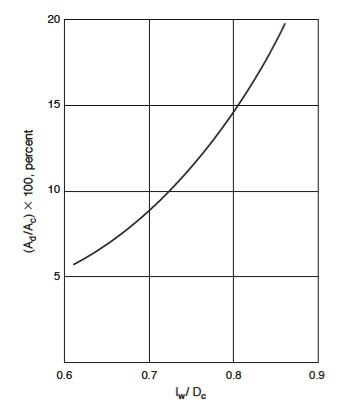
Standard sizes for trays -- and good assumptions for the first iteration -- are: weir height, h<sub>w</sub> = 50mm ; hole diameter, D<sub>h</sub> = 5mm ; plate thickness, t<sub>pl</sub> = 5mm. From the graph below, the ratio of downcomer area (A<sub>d</sub>) to column cross-sectional area (A<sub>c</sub>) can be determined from the ratio of weir length (l<sub>w</sub>) to column diameter (D<sub>c</sub>) and vice versa. | |||
[[File:Fig1133.jpg|200px|center|frame|Figure 7: Relation between downcomer area and weir length.]] | |||
=====7. Check the weeping rate===== | |||
Compare the actual vapor velocity to the minimum vapor velocity -- if velocity is too low fluid will "weep" through the tray holes. If the weeping rate is unsatisfactory, return to step 6 and choose different values for the plate layout dimensions. From the chart in step 4, it can be seen that there is a minimum vapor flow rate below which the liquid "weeps" from the tray above. | |||
For the remaining steps in this design process, it is recommended to check your assumptions after each step and revise them as necessary in order to maintain operation in the "sweet spot" of the vapor rate vs. liquid rate plot. Additional iterations may be required as you move through the procedure. | |||
[[File:Fig1132.jpg|200px|center|frame|Figure 8: Weep-point corrlelation.]] | |||
Calculate the maximum liquid flow rate. Calculate the minimum liquid flow rate at 70% turndown (recommended). Calculate the height over the weir as | |||
<math>h_o=750[\frac{L_w}{p_Ll_w}]^\frac{2}{3}</math> | |||
=====8. Check the plate pressure drop===== | |||
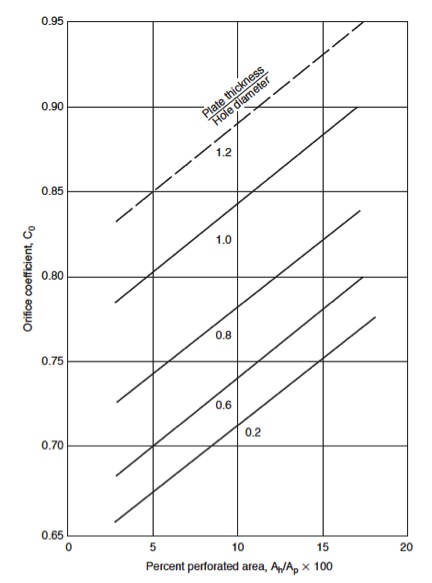
[[File:Fig1136.jpg|50px|right|frame|Figure 9: Percent perforated area, A<sub>h</sub>/A<sub>p</sub> x100]] | |||
<dfn>If the pressure drop calculated here is too high, return to step 6.</dfn> | |||
Proceed to step 9 if the pressure drop assumption is valid. | |||
=====9. Check the downcomer backup. ===== | |||
<dfn>If the downcomer backup is too high, return to step 6 or 3.</dfn> | |||
The plate spacing affects the amount of fluid in the downcomer. Calculate the level in the downcomer and the residence time of the fluid to see if the values are valid. Note that residence times greater than 3 seconds are acceptable. | |||
Proceed to step 10 if residence time is acceptable. | |||
=====10. Decide plate layout details.===== | |||
Determine calming zones, the unperforated areas at the inlet and outlet sides of the plate. The width of each zone is usually made the same. Recommended values are: below 1.5 m diameter, 75 mm; above, 100 mm. The unperforated area can be calculated from plate geometry. Also check the hole pitch, or the distance between hole centers. It should not be less than 2.0 hole diameters. A normal range is between 2.5 and 4.0 hole diameters. The shape must also be specified. Square and equilateral triangle holes are used. | |||
=====11. Recalculate the percentage flooding based on the chosen column diameter.===== | |||
An assumption of 80% flooding was chosen so that operation would occur in the "sweet spot." This assumption must be checked by calculating the flooding percentage for a given column diameter. | |||
u<sub>v</sub> = (max volumetric flow rate)/(net area) | |||
<math>%flooding = \frac{u_v}{u_f}</math> | |||
If the hole pitch is unsatisfactory, return to step 6. | |||
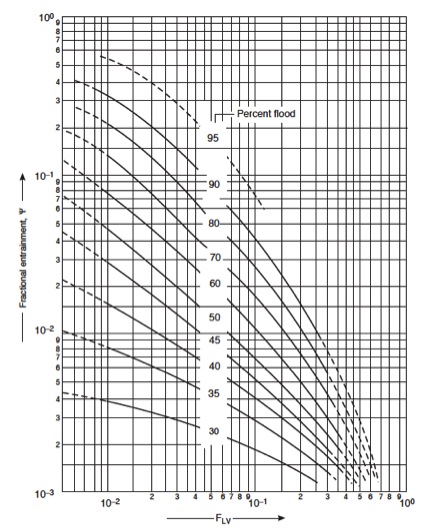
=====12. Check entrainment===== | |||
''If too high, return to step 4'' Use the graph below to determine entrainment from F<sub>LV</sub>. | |||
[[File:Fig1131.jpg|300px|center|frame|Figure 10: Entrainment correlation for sieve plates]] | |||
The value for fractional entrainment can be used to re-estimate the column efficiency, and reevaluate the number of trays needed. Can return to step 1 for more accurate estimates. | |||
=====13. Optimize design.===== | |||
After returning to step 1 to reevaluate the number of trays, it is valuable to repeat steps 2 through 12 to find the smallest diameter and plate spacing acceptable at the lowest cost. | |||
=====14. Finalize the design.===== | |||
Optional: draw up the plate specification and sketch the layout of the plate. | |||
====Bubble-Cap Trays==== | ====Bubble-Cap Trays==== | ||
| Line 52: | Line 180: | ||
====Flow Patterns==== | ====Flow Patterns==== | ||
Cross flow columns are the most common pattern for distillation columns. For liquid flows between 50 and 500 Gal/min, a cross flow column is appropriate. When liquid flow is increased above 500 Gal/min, an engineer should consider designing a double pass or multi-pass column. This will reduce the liquid gradient on the tray and reduce the downcomer loading | Cross flow columns are the most common pattern for distillation columns. For liquid flows between 50 and 500 Gal/min, a cross flow column is appropriate. When liquid flow is increased above 500 Gal/min, an engineer should consider designing a double pass or multi-pass column. This will reduce the liquid gradient on the tray and reduce the downcomer loading (Wankat, 2012). | ||
===Column Sizing=== | ===Column Sizing=== | ||
| Line 69: | Line 197: | ||
<math>D_c = \sqrt{\frac{4\hat{V_w}}{\pi\rho_v\hat{u_v}}}</math> | <math>D_c = \sqrt{\frac{4\hat{V_w}}{\pi\rho_v\hat{u_v}}}</math> | ||
where <math>\hat{V_w}</math> is the maximum vapor rate in kg/s | where <math>\hat{V_w}</math> is the maximum vapor rate in kg/s (Towler et al., 2013). | ||
===Distillation Applications=== | ===Distillation Applications=== | ||
| Line 75: | Line 203: | ||
Distillation is a process that can be implemented in various scales. There is both laboratory scaled distillation as well as very large industrial distillation. Other applications for distillation include food/alcohol processing and herb distillation for the perfume and medical industries. Typically laboratory scaled distillation occurs in batches whereas industrial distillation (e.g. fractional distillation of crude oil) occurs continuous with a constant distillate and bottom effluent streams. | Distillation is a process that can be implemented in various scales. There is both laboratory scaled distillation as well as very large industrial distillation. Other applications for distillation include food/alcohol processing and herb distillation for the perfume and medical industries. Typically laboratory scaled distillation occurs in batches whereas industrial distillation (e.g. fractional distillation of crude oil) occurs continuous with a constant distillate and bottom effluent streams. | ||
Some applications of distillation are concerned the top stream only, some the bottom stream only and others both streams can be used for future products. In alcohol distillation for example, the water that is separated from the ethanol/water binary solution is discarded as waste water. In fractional distillation of crude oils, the heavy hydrocarbons at the bottom of the column are collected and sold along with the light hydrocarbons that appear in higher side draws | Some applications of distillation are concerned the top stream only, some the bottom stream only and others both streams can be used for future products. In alcohol distillation for example, the water that is separated from the ethanol/water binary solution is discarded as waste water. In fractional distillation of crude oils, the heavy hydrocarbons at the bottom of the column are collected and sold along with the light hydrocarbons that appear in higher side draws (Wankat, 2012). | ||
===Example Case: Ideal Distillation=== | ===Example Case: Ideal Distillation=== | ||
| Line 123: | Line 251: | ||
where <math>\alpha_{ik}</math> is the relative volatility of species <math>i</math> to species <math>k</math>, <math>f_i</math> the molar flow of species <math>i</math> in the feed, <math>q</math> the fraction of the feed that joins the liquid stream at the feed tray, <math>F</math> the total molar flow of the feed, <math>D</math> the molar flow of the distillate, <math>R_{min}</math> the minimum reflux ratio <math>(=L_{min}/D)</math>, <math>d_i</math> the molar flow of species <math>i</math> in the distillate, <math>V_{min}</math> the minimum vapor flow possible in the top section of the column to accomplish the desired separation, <math>\bar R_{min}</math> the minimum reboil ratio <math>(=\bar V_{min}/B)</math>, <math>b_i</math> the molar flow of species <math>i</math> in the bottoms product, and <math>\bar V_{min}</math> the minimum vapor flow in the bottom section of the column. The final variable, <math>\phi</math>, will be solved for using the first Underwood equation, and it's value will be decided based on the relative volatilities of the key components in the column. | where <math>\alpha_{ik}</math> is the relative volatility of species <math>i</math> to species <math>k</math>, <math>f_i</math> the molar flow of species <math>i</math> in the feed, <math>q</math> the fraction of the feed that joins the liquid stream at the feed tray, <math>F</math> the total molar flow of the feed, <math>D</math> the molar flow of the distillate, <math>R_{min}</math> the minimum reflux ratio <math>(=L_{min}/D)</math>, <math>d_i</math> the molar flow of species <math>i</math> in the distillate, <math>V_{min}</math> the minimum vapor flow possible in the top section of the column to accomplish the desired separation, <math>\bar R_{min}</math> the minimum reboil ratio <math>(=\bar V_{min}/B)</math>, <math>b_i</math> the molar flow of species <math>i</math> in the bottoms product, and <math>\bar V_{min}</math> the minimum vapor flow in the bottom section of the column. The final variable, <math>\phi</math>, will be solved for using the first Underwood equation, and it's value will be decided based on the relative volatilities of the key components in the column. | ||
So, after solving the first Underwood equation, we get two values for <math>\phi</math>, 3.806 and 1.462. Because 3.806 is between the relative volatilities of the key components, we will substitute that value for <math>\phi</math> into the second Underwood equation. Doing so for both columns gives <math>V_{min} = 6.4\ mol/s</math> for the first column and <math>V_{min} = 8.9\ mol/s</math> for the second column, for a total minimum vapor flow of 15.3 mol/s. The process would then be repeated for the indirect sequence, and the decision for which process to use would be justified by the process with the overall minimum vapor flow | So, after solving the first Underwood equation, we get two values for <math>\phi</math>, 3.806 and 1.462. Because 3.806 is between the relative volatilities of the key components, we will substitute that value for <math>\phi</math> into the second Underwood equation. Doing so for both columns gives <math>V_{min} = 6.4\ mol/s</math> for the first column and <math>V_{min} = 8.9\ mol/s</math> for the second column, for a total minimum vapor flow of 15.3 mol/s. The process would then be repeated for the indirect sequence, and the decision for which process to use would be justified by the process with the overall minimum vapor flow (Biegler et al., 1997). | ||
==Absorption== | ==Absorption== | ||
| Line 133: | Line 261: | ||
<math>A_i=L/K_iV</math> | <math>A_i=L/K_iV</math> | ||
where <math>L</math> is the liquid flow rate entering the column, <math>V</math> is the vapor flow rate entering the column, and <math>K_i</math> is the vapor/liquid equilibrium ratio for component i | where <math>L</math> is the liquid flow rate entering the column, <math>V</math> is the vapor flow rate entering the column, and <math>K_i</math> is the vapor/liquid equilibrium ratio for component i (Peters & Timmerhaus, 2003). Higher absorption factors result in higher absorptivity into the liquid and a decrease in the number of trays required for separation, however a diminishing return occurs after the absorption factor is greater than 2.0. An absorption factor of 1.4 is most commonly used. | ||
In general absorption can be seperated into two overarching categories, physical and chemical absorption. In physical absorption, the unwanted solute in the gas is absorbed into the liquid phase because solubility of the component is higher in the liquid phase than the gas phase. In chemical absorption the solute is removed from the gas via a reaction with the solvent, this reacted product is then transported into the liquid phase | In general absorption can be seperated into two overarching categories, physical and chemical absorption. In physical absorption, the unwanted solute in the gas is absorbed into the liquid phase because solubility of the component is higher in the liquid phase than the gas phase. In chemical absorption the solute is removed from the gas via a reaction with the solvent, this reacted product is then transported into the liquid phase (Danckwerts 1965). There are two types of chemical absorption reversible and irreversible. Generally reversible chemical absorption is preferred as the solvent can be put through a stripper and regenerated so it can be recycled back to the absorption process (Wankat, 2012). | ||
===Absorption Apparatus=== | ===Absorption Apparatus=== | ||
[[File: | There are five major apparatus used for absorption in industrial application. These five pieces of equipment are spray absorbers (or towers), ejector (venturi) scrubbers, packed columns, trayed columns, and film absorbers (Schmidt, 2012). | ||
Spray towers on the other hand generally have many nozzle at different heights where the liquid solvent will be sprayed out of to contact the gas running through the tower. This design is used in order to ensure the gas contacts the liquid as throughout the tower. These nozzles are lower pressure than a | |||
==== Spray Tower vs Ejector Scrubber ==== | |||
In both '''spray tower''' and the '''ejector scrubber''' nozzles are employed to produce small solvent droplets. These small droplets increase the surface area of the liquid to gas contact allowing for the maximum amount of mass transfer to occur between the gas mixture and the liquid. The major difference between the two nozzle equipment designs is the configuration and type of nozzles. In the ejector scrubber shown in Figure 11 there is a single nozzle that is generally a higher pressure spray nozzle that produces finer solvent drops allowing for an even greater amount of mass transfer enabling better physical absorption (Schmidt, 2012). | |||
[[File:Ejectorventuri.jpg|thumb|200px|center|Figure 11. Ejector Scrubber (US EPA, 2006)]] | |||
'''Spray towers''' on the other hand generally have many nozzle at different heights where the liquid solvent will be sprayed out of to contact the gas running through the tower. This design is used in order to ensure the gas contacts the liquid as throughout the tower. These nozzles are lower pressure than a ejector scrubbers nozzle and thus physical mixing is worse in this configuration. Since physical mixing is generally worse in this configuration it is usually used in conjunction with a chemical absorption process. The other major difference between the ejector scrubber and the spray tower is that gas and liquid flow is cocurrent in the former while it is countercurrent in a spray tower. A spray tower absorber is shown below in Figure 12 (Schmidt, 2012). | |||
[[File:SparyTowerAbsorber.jpg|thumb|200px|center|Figure 12. Spray Tower Absorber (US EPA, 2006)]] | |||
==== Tower Type Absorption Apparatus ==== | |||
'''Packed column absorbers''' and '''tray column absorbers''' have very high efficiencies for the removal of an unwanted solute in the gas stream. The major disadvantage a trayed column has when compared to a packed column is the pressure drop. The pressure drop in a packed column is generally very low, whereas in between each tray of a trayed column pressure drop can be quite large. However the advantages inherent to trayed columns become clear when one needs the solvent to have a high concentration of the component to be removed from the gas stream. This is most important in the case where there is a very low concentration of the component in the gas stream and the specification states the solvent must contain a high concentration of that component. In this case the flow rate of the solvent may not be high enough for a packed column, however in a trayed column the solvent flow rate can be near zero for operation (Schmidt, 2012). Packed and trayed column internals are very similar to the setups found in the respective distillation columns. | |||
For a '''trayed column''' the plate efficiency can be calculated using O'Connell's Correlation which invovles the Henry's Law constant, total system pressure, and solvent viscosity at the operating temperature (Towler & Sinnott, 2013). | |||
<math>x=0.062*\frac{\rho_s*P}{\mu_s*H*M_s}</math> | |||
where | |||
<math>x</math> is the tray efficiency, | |||
<math>\rho_s</math> is the density of the solvent in <math>kg/m^3</math>, | |||
<math>P</math> is the total pressure of the system in <math>N/m^2</math>, | |||
<math>\mu_s</math> is the solvent's viscosity in <math>mNs/m^2</math>, | |||
<math>H</math> is the Henry Law constant in <math>1/(Nm^2*(mol fraction))</math>, | |||
and <math>M_s</math> is the molecular weight of the solvent. | |||
A packed towers height can be determined using the equations below when concentration of solute is below 10% so that the assumption that the flow of gas and liquid will be essentially constant throughout the column holds (Towler & Sinnott, 2013). The height of packing <math>Z</math> is given by the following equation: | |||
<math>Z=\frac{L_m}{K_G*a*P}*\int\limits_{y_2}^{y_1} \frac{dy}{y-y_e}\,</math> | |||
where <math>P</math> is the total pressure, <math>a</math> is the interfacial surface area per unit volume, <math>y_1</math> and <math>y_2</math> are the mol fractions of the solute in the gas stream at the bottom and top of the column respectively, <math>G_m</math> is the molar gas flow rate per unit cross-sectional area, and <math>y_e</math> is the mole fraction of solute in the gas that would be in equilibrium with the liquid concentration. | |||
The first half of the equation before the integral can be called the height of an overall gas-phase transfer unit <math>H_G</math> and the second part of the equation is the number of overall gas-phase transfer units or <math>N_G</math>. Using these definitions the above equation can be simplified to | |||
<math>Z=H_G*N_G</math> | |||
The final absorber the film absorber is generally used in the case where the heat of absorption must be removed. The film absorber operates by sending the gas and solvent through a heat exchanger where the solvent creates a thin film on the walls of the tubes and the gas flows through the interior allowing for solute transfer. The good heat transfer present in a film absorber makes it preferable for situations where low temperatures are required for a high recovery of the solute | These equations assist in sizing an absorption column (Towler & Sinnott, 2013). | ||
==== Film Absorber ==== | |||
The final absorber the film absorber is generally used in the case where the heat of absorption must be removed. The film absorber operates by sending the gas and solvent through a heat exchanger where the solvent creates a thin film on the walls of the tubes and the gas flows through the interior allowing for solute transfer. The good heat transfer present in a film absorber makes it preferable for situations where low temperatures are required for a high recovery of the solute (Schmidt 2012). | |||
===Industrial Absorption Processes=== | ===Industrial Absorption Processes=== | ||
An industrial example is lean oil absorption, which is used to separate nitrogen and other impurities from natural gas. A lean oil is contacted with low quality natural gas, and the methane is selectively absorbed by the lean oil, leaving the impurities behind. The methane is subsequently regenerated from the rich oil as high quality natural gas | An industrial example is lean oil absorption, which is used to separate nitrogen and other impurities from natural gas. A lean oil is contacted with low quality natural gas, and the methane is selectively absorbed by the lean oil, leaving the impurities behind. The methane is subsequently regenerated from the rich oil as high quality natural gas (Petrogas Systems, 2014). | ||
Other common industrial practices of absorption come from sour gas treatment. Amine gas treating is used to remove hydrogen sulfide or carbon dioxide from gas streams via a reversible chemical absorption. In amine gas treating the sour gas is fed to the bottom an absorber where amine solution is fed to the top along with any necessary make up water. The sour gas components are absorbed into the amine via a chemical absorption method. Sweet gas leaves the top of the absorber whereas the amine out of the bottom, now rich with acidic components is sent to a regenerator where the acid gas components are stripped and the acid gas is generally sent to a flare whereas the amine now lean again is recycled back into the first absorber | Other common industrial practices of absorption come from sour gas treatment. Amine gas treating is used to remove hydrogen sulfide or carbon dioxide from gas streams via a reversible chemical absorption. In amine gas treating the sour gas is fed to the bottom an absorber where amine solution is fed to the top along with any necessary make up water. The sour gas components are absorbed into the amine via a chemical absorption method. Sweet gas leaves the top of the absorber whereas the amine out of the bottom, now rich with acidic components is sent to a regenerator where the acid gas components are stripped and the acid gas is generally sent to a flare whereas the amine now lean again is recycled back into the first absorber (Miller & Zawacki, 1978). Figure 13 below shows the typical setup of an amine plant. Another type of sour gas treatment that uses absorption is Merichems LO-CAT process which uses a chelated iron to remove hydrogen sulfide from feed gas in the absorption column (Merichem 2015). | ||
[[File:AmineTreating.png|thumb|400px|center|Figure 13. Amine Gas Treating Plant Schematic]] | |||
==Stripping== | ==Stripping== | ||
| Line 160: | Line 319: | ||
<math>S_i=K_iV/L</math> | <math>S_i=K_iV/L</math> | ||
where <math>K_i</math> is the vapor/liquid equilibrium ratio, <math>V</math> is the vapor flow rate entering the column, and <math>L</math> is the liquid flow rate entering the column, will determine how much of solute i will be stripped from the liquid into the vapor phase | where <math>K_i</math> is the vapor/liquid equilibrium ratio, <math>V</math> is the vapor flow rate entering the column, and <math>L</math> is the liquid flow rate entering the column, will determine how much of solute i will be stripped from the liquid into the vapor phase (Peters & Timmerhaus, 2003). The usual range for the stripping factor is between 1.2 and 2.0, with a stripping factor of 1.4 being most economic. | ||
An example of stripping in industry is the deodorization of food items such as oils. The oil is heated and allowed to trickle down the column while steam flows up from the bottom of the column. At the vapor-liquid interface, volatile components of the oil transfer to the steam and are carried off the top of the column, leaving a purified oil product | An example of stripping in industry is the deodorization of food items such as oils. The oil is heated and allowed to trickle down the column while steam flows up from the bottom of the column. At the vapor-liquid interface, volatile components of the oil transfer to the steam and are carried off the top of the column, leaving a purified oil product (Alfa Laval, 2014). | ||
==Bioseparations== | ==Bioseparations== | ||
===Importance=== | ===Importance=== | ||
As our ability to manipulate and engineer biological systems improves, biological products are becoming an increasingly important source of therapeutics and fuels. The production of fuels from biomass via either the enzymatic breakdown of a feedstock or the secretion of usable lipids from algae is a promising new energy source. Additionally, enzymes, antibodies and other therapeutic proteins have been applied to the treatment of a wide range of diseases. Although each process requires its own set of separations, all follow the same basic format: separation of biomass, product isolation, and product purification. This section will provide examples of unit operations in each step | As our ability to manipulate and engineer biological systems improves, biological products are becoming an increasingly important source of therapeutics and fuels. The production of fuels from biomass via either the enzymatic breakdown of a feedstock or the secretion of usable lipids from algae is a promising new energy source. Additionally, enzymes, antibodies and other therapeutic proteins have been applied to the treatment of a wide range of diseases. Although each process requires its own set of separations, all follow the same basic format: separation of biomass, product isolation, and product purification (Belter et al., 1998). This section will provide examples of unit operations in each step. Ultimately, the choice of separation process and unit operations will depend on the specific process and product. The descriptions below are examples of the most common bioseparation operations within the general platform (Harrison et al., 2003). | ||
Bioprocesses begin with fermentations or growth operations. In biofuel production processes, this may involve growing algae or breaking down corn or cellulosic biomass. For the production of therapeutics, mammalian or bacterial cells may be grown in a fermentor and the product secreted into the supernatant or harvested from the cells. | Bioprocesses begin with fermentations or growth operations. In biofuel production processes, this may involve growing algae or breaking down corn or cellulosic biomass. For the production of therapeutics, mammalian or bacterial cells may be grown in a fermentor and the product secreted into the supernatant or harvested from the cells. | ||
| Line 172: | Line 331: | ||
===Biomass Separations=== | ===Biomass Separations=== | ||
After fermentation and product production, the solid biomass must first be separated from the desired product. If the product is secreted from the cells, this can be done immediately after fermentation ends. If the product is not secreted, the cells must first be lysed. | After fermentation and product production, the solid biomass must first be separated from the desired product. If the product is secreted from the cells, this can be done immediately after fermentation ends. If the product is not secreted, the cells must first be lysed. | ||
Cell lysis is the process of lysing, or breaking, the cell in open. Mechanical lysis is the simplest, and involves physically breaking the cell either by mashing (think mortar and pestle) or blending the cells into a homogenous solution. Chemical lysis is another method, achieved by introducing an osmotic shock or chemically degrading the cell membrane. Additional separation can be achieved by flocculation, which is the process of aggregating biomaterial by charge neutralization or bridging. These larger complexes are easier to separate from smaller molecules [ | Cell lysis is the process of lysing, or breaking, the cell in open. Mechanical lysis is the simplest, and involves physically breaking the cell either by mashing (think mortar and pestle) or blending the cells into a homogenous solution in a homogenizer. Chemical lysis is another method, achieved by introducing an osmotic shock or chemically degrading the cell membrane. Additional separation can be achieved by flocculation, which is the process of aggregating biomaterial by charge neutralization or bridging. These larger complexes are easier to separate from smaller molecules (Harrison et al., 2003). | ||
The next step is removing the unwanted biomass from the product in solution. Separation by centrifugation or sedimentation are the most common, although filtration is sometimes also used for processes where a biomass cake is desired. Both methods utilize density differences to separate the product from the solid biomass (Towler and Sinnott, 2013). | |||
====Sedimentation==== | |||
Sedimentation relies purely on the force of gravity, while centrifugation speeds the settling process by subjecting the cells to a centrifugal force. Sedimentation in a settling tank is the simplest method of solid-liquid bioseparation. In this process, biomass in a tank is simply allowed to settle to the bottom over time. While this process is inexpensive, requires little energy and can separate out large volumes of biomass, it generally requires long time periods and is only mostly in very large-scale processes where active centrifugation is difficult (Belter et al., 1998). | |||
====Centrifugation==== | |||
Centrifuges are widely utilized across many processes, and thus a wide variety of scales and designs have been developed. <i> Disk-stack centrifuges</i>, in which the solid phase is deposited onto “shelves” in the center of the spinner and liquid phase is pushed to the outside, are some of the most commonly used centrifuges in industry. They are especially suited to biomass separation processes because they can be built on a large scale and are ideal for separating fine solids from liquids. [[File: Disk_stack_centrifuge_towler.png|frame|center|Fig. 14: Diagram of a disk-stack centrifuge (Tolwer et al, 1997).]] <i>Tubular bowl centrifuges</i> are also common and can reach separation efficiencies of up to 90%. Heavier products accumulate along the sides of the bowl, while the light phase flows out the top. They separate products by can be used both to separate solids from liquids and immiscible liquids, such as and oil product and an aqueous broth (Tolwer and Sinnott, 2013). [[File: tubular bowl centrifuge towler.png|frame|center|Fig. 15: Diagram of a tubular bowl centrifuge centrifuge (Tolwer and Sinnott, 2013).]] | |||
Centrifugation scale-up is made easier by <i>sigma analysis</i>, which allows for the estimation of appropriate feed rates for different size centrifuges. The sigma factor is dependent on the inner and outer radius of the centrifuge, the angular velocity, and the sedimentation velocity of the solid particles being separated. It can be thought of as the characteristic cross-sectional area with units of [length]<sup>2</sup>. The sedimentation velocity can be calculated by | |||
<math>v_g={\frac{2a^2(\rho-\rho_0)}{9\mu}}g</math> | |||
where <math>v_g</math> is the sedimentation velocity, <math>a</math> is the cell or biomass particle diameter, <math>\rho</math> is the particle density, <math>\rho_0</math> is the fluid density, and <math>\mu</math> is the fluid viscosity. The volumetric flow <math>Q</math> can be estimated by | |||
<math>Q=(v_g)(\Sigma)</math>. | |||
The equality | |||
<math>{\frac{\Sigma_1}{\Sigma_2}}={\frac{Q_1}{Q_2}}</math> | |||
can be an easy way to estimate equivalent flow rates between a small-scale centrifuge 1 and larger centrifuge 2 (Harrison et al., 2003). | |||
====Example: Centrifugation Scale-up==== | |||
The | You are trying to separate a cell of radius 0.4 <math>\mu</math>m with a density of 1.05 g/cm<sup>3</sup> from broth of mostly water (density of 1 g/cm<sup>3</sup> and viscosity of 0.01 g/cm s). The sigma factor of the centrifuge you are using is 1 x 10<sup>6</sup> cm<sup>2</sup>. A] What volumetric flow rate should you use? B] If you want to scale up the process to a centrifuge with <math>\Sigma</math> = 3 x 10<sup>6</sup> cm<sup>2</sup>, what flow rate would you use in the larger centrifuge? | ||
< | Solution: | ||
A] Using the equation for <math>v_g</math>, and being mindful of units, the sedimentation velocity equals 1.74 x 10<sup>6</sup> cm/s. The flow rate, then, equals | |||
< | <math>Q=(1.74 x 10^-6)(1,000,000) = 1.74 cm^3/s = 0.104 L/min</math>. | ||
B] Keeping in mind that for the same process, <math>v_g1 = v_g2,</math> and rearranging the sigma factor equality, the new flow rate is | |||
<math>Q_2 = {\frac{\Sigma_2 x Q_1}{\Sigma_1}} = {\frac{(3 x 10^6)(0.104)}{1 x 10^6}} = 0.313 L/min </math> | |||
===Product Isolation=== | ===Product Isolation=== | ||
Liquid-liquid separation, to extract the product from the aqueous phase, is much less straightforward than liquid-solid extraction. Many methods - especially | Liquid-liquid separation, to extract the product from the aqueous phase, is much less straightforward than liquid-solid extraction. Many methods - especially adsorption, filtration, and precipitation - are similar in principle to operations found in other, non-biological separations. The exact separations used depend on the nature of the product and the scale of the process. These processes are nearly identical to their non-biological counterparts, and their description is left to other sections. | ||
Particular care needs to be taken with protein products because of their instability, and the selection of an appropriate solvent or adsorbent is crucial to a successful process (Harrison et al., 2003). | |||
===Product Purification=== | ===Product Purification=== | ||
The final steps of protein purification and polishing remove any remaining contaminants and bring the concentration of product to an appropriate value for applications. Purification processes for medical | The final steps of protein purification and polishing remove any remaining contaminants and bring the concentration of product to an appropriate value for applications. Purification processes for food-grade and medical products can be extensive, as sterility and high purity are essential. Purification in fuel-producing processes may be less extensive, depending on the process. Chromatography and crystallization are two common steps in purification and are especially used in industrial scale protein production. Several different types of chromatography exist with the ability to carry out different types of separations. | ||
Chromatography is similar to adsorption in that it relies on differences in affinity between solutes and a solid surface. A solution is eluted through a column containing a solid resin with various affinities for the substances in solution. In adsorption, the solutes are evenly saturated throughout the column. Chromatography differs in that solutes are deposited a resin phase before the column is flushed with an elution solvent specific that results in solutes eluted in bands. | |||
==== Ion Exchange Chromatography ==== | |||
There are two main types of ion exchange columns—anion and cation. Anion exchange resins have a positive charge and are used to retain products with a negative charge. Cation exchange resins have a negative charge and are used to retain products with a positive charge. The pH of the elution buffer is change to force a specific solute to wash out, depending on whether the pH of the buffer is above or below the isoelectric point of the solute (Belter et al., 1998). This is especially useful for the separation of protein product (including antibodies), nucleic acids, and other charged molecules. When the solutes have sufficiently different isoelectric points, the pH of the buffer is manipulated to affect the solute charge and force the product to elute while the solute remains preferentially bound to the resin, or vice versa (Harrison et al., 2003). In general, the most strongly charged molecules will remain in the column for a longer period of time. Elution washes through the weakly bound ions before the more strongly bound ions. Different speeds of elution can be visualized as in figure 16. | |||
[[File:chromatography.png|frame|center|Fig. 16: Illustration of product bands in an elution chromatography column (Belter et al., 1998).]] | |||
Chromatography | ==== Size Exclusion Chromatography ==== | ||
Crystallization, or the formation of solute crystals from a solution, is especially useful in biomolecule separations because it is possible to obtain a 99.9%+ product purity. In crystallization, a diluent is added to the homogeneous solution that reduces the solubility of the product to the point that it “falls out” of solution and crystallizes. It is similar to precipitation but results in the formation of crystals rather than unordered aggregates.Crystallization can be used on a laboratory scale for determining protein structure, on on the industrial scale for antibody and therapeutic protein productions. Batch crystallizers are often used in industry because of their simplicity and inexpensiveness compared to continuous crystallization [ | In gel filtration chromatography, small molecules are "trapped' by the porous resin and take longer to flow through the column. Larger products will elute first because the smaller molecules are better able to penetrate the resin. This forces them to take a much longer path through the column, which means it takes longer for them to elute. This operation is often used when there is a distinct difference in size between the desired product and other solutes. | ||
==== Affinity Separations ==== | |||
Affinity chromatography is very similar to ion exchange chromatography in that the interactions between the material in the column and the molecules in the feed. The main difference is that affinity chromatography can rely on a great variety of types of interactions. Two very common types of affinity are exploited in affinity chromatography columns. The first is immunoaffinity. Proteins are specifically bound by antibodies which can be incorporated onto beads and used in chromatography. Antibodies are designed to bind only a single protein, so these interactions are considered to be highly specific. The protein can be eluted using a buffer that changes the pH or salinity in the column, which adversely affects binding (Hage, 1999). | |||
Proteins can be separated from very complex and unrefined mixtures using this method because of the great specificity. Antibody-protein interactions are so specific that individual proteins can be isolated from blood samples as shown in the figure below. The main drawback of this method is simply that specific interactions between proteins and binding targets do not always exist. Antibodies exist for many proteins, but for others they must be customized or created using some sort of evolution process. This is not a trivial task and can require a significant capital investment (Hage, 1999). | |||
[[File:Affinity_Chromatography_Example.PNG|frame|center|Fig. 17: Affinity purification separating fibrinogen from human plasma using an anti-fibrinogen antibody (Hage, 1999).]] | |||
The other main type of affinity chromatography is based on protein specific tags and the molecules or surfaces to which they bind. One of the most common types of protein tags used is the polyhistidine tag. This tag consists of 6-8 consecutive histidine residues which can be added to the exterior of the desired protein product. The addition of this tag requires alterations to the coding sequence of the protein. The polyhistidine tag binds strongly with nickel and cobalt ions. The product with the tag can then be eluted with imidazole—a small molecule with the same structure as the functional group of the amino acid histidine. Imidazole will bind the cobalt and nickel ions more strongly than the histidine in the tag. Along with chromatography, protein tag interactions can be leveraged with the use of beads that can be deposited directly into the solution containing the protein of interest (Lichty et al., 2005). | |||
Different proteins can be purified in this manner with varying levels of efficiency. There is a very high dependence on the size of the protein, electronic properties, and steric considerations. The figure below demonstrates varying separation efficiencies with proteins of different sizes (HisPur Cobalt Resin - Thermo Fisher Scientific, 2016). | |||
[[File:HisTagPurification.PNG|frame|center|Fig. 18: Protein gel demonstrating the separation of proteins from a sample. The far left column represents the most efficient separating conditions using cobalt and the far right column represents a negative control with no purification performed (HisPur Cobalt Resin - Thermo Fisher Scientific, 2016).]] | |||
Several other types of tag-bead interactions can be utilized in separations processes. Maltose Binding Protein is a small protein that can be added to a protein of interest. It binds strongly with beads coated in immobilized maltose and can be released by flushing with maltose. As MBP is a full sized protein that typically must be removed from the protein of interest in order for it to be used. In this case, the site specific TEV protease is often used cleave MBP from the protein of interest. In addition, under specific circumstances, other unique tags can be used and provide varying levels of specificity in separations. The Flag tag, 3x Flag tag, Glut tag, and Strep tag. While these are all commonly used, the polyhistidine tag is the most popular because it gives the highest level of specificity. Some of the main purification tags are compared in the table below (Lichty et al., 2005). | |||
{| class="wikitable" | |||
|- | |||
! Tag | |||
! Size (aa) | |||
! Resin | |||
! Eluting Agent | |||
! Capacity (mg/ml) | |||
! Cost/10 mg | |||
|- | |||
| His | |||
| 6 | |||
| Talon | |||
| Imidazole | |||
| 5-14 | |||
| $18 | |||
|- | |||
| Maltose Binding Protein | |||
| 396 | |||
| Amylose | |||
| Maltose | |||
| 3 | |||
| $12 | |||
|- | |||
| Glutathione S-Transferase | |||
| 218 | |||
| GSH-Sepharose | |||
| Glutathione | |||
| 10 | |||
| $36 | |||
|- | |||
| Strep II | |||
| 8 | |||
| Strep-Tactin Sepharose | |||
| Desthiobiotin | |||
| 0.1 | |||
| $293 | |||
|- | |||
| FLAG | |||
| 8 | |||
| Anti-FLAG M2 MAb Agarose | |||
| Flag peptide | |||
| 0.6 | |||
| $1045 | |||
|} | |||
Unfortunately, based on the contents of the solution, the characteristics of the protein, and the specific product being used, there are extremely large amounts of variation in yield and purity for each type of tag. Therefore, it is crucial to allocate time towards preliminary tests and scale-up experiments with the exact solution from which the protein of interest is being separated. The his tag is typically the most used because, as seen in the table above, it has one of the lowest costs and highest yields. In addition, because it is only a 6 amino acids, it often is not cleaved from the protein of interest which minimizes the number of steps required in the overall purification process (Lichty et al., 2005). | |||
==== Crystallization ==== | |||
Crystallization, or the formation of solute crystals from a solution, is especially useful in biomolecule separations because it is possible to obtain a 99.9%+ product purity. In crystallization, a diluent is added to the homogeneous solution that reduces the solubility of the product to the point that it “falls out” of solution and crystallizes. It is similar to precipitation but results in the formation of crystals rather than unordered aggregates. Crystallization can be used on a laboratory scale for determining protein structure, on on the industrial scale for antibody and therapeutic protein productions. Batch crystallizers are often used in industry because of their simplicity and inexpensiveness compared to continuous crystallization (Harrison et al., 2003). | |||
==Membrane Separation== | |||
Membrane separation takes advantage of the selective permeability of membranes; they allow certain particles to pass through and selectively stop other, generally unwanted, particles. The component that passes through is called the permeate and the component stream that is rejected is called the retentate or concentrate. The applicability of membranes comes from the fact that their selectivity is determined by their pore size, which can be controlled during the creation of the membranes. Additionally, Membrane processes do not require heat meaning they generally require less energy than conventional separations technology such as distillation and crystallization. Membrane separations processes are generally classified as microfiltration, ultrafiltration, or nanofiltration depending on the size of the particles to be filtered out. | |||
[[File:membrane techs.png|frame|center|Figure 19. Cutoffs for different membrane categories]] | |||
===Membrane Selection, Construction, and Flow Geometries=== | |||
Membrane permeability and selectivity are the two most important factors to consider when selecting a membrane. For gas separations, the permeation of the gas is usually facilitated by the gas dissolving in the membrane on one side and then evaporating on the permeate side. Therefore permeability depend largely on the solubility of components in the membrane. | |||
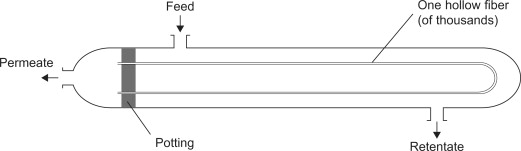
The two most commonly utilized membrane configurations are hollow fiber and spiral wound. Hollow fiber is generally the most commonly utilized module for gas separations. These are formed by gluing the two ends of the hollow fiber to a resin forming a closure. The fibers are housed in a shell much like a heat exchanger. The feed flows past thousands of tubes with the permeate flowing into the hollow tubes and out the closure. The retentate then flows out of the shell not having gotten through the membrane. | |||
[[File:hollow membrane.jpg|frame|Figure 20. Hollow fiber membrane module]] | |||
Spiral wound membranes are created by sealing two membrane sheets back to back on three edges to form a sort of pocket. This fourth open edge is then attached to a porous tube which allows permeate to go through it. Several membrane pockets are attached to a single tube and wrapped around in a spiral. | |||
[[File:spiral membrane.jpg|frame|Figure 21. Spiral wound membrane module]] | |||
Flow geometry is usually either dead-end geometry or cross flow geometry. In dead end, the fluid flow is normal to the membrane surface while cross flow is parallel to the membrane surface. Dead end geometry is usually used with hollow fiber membranes while cross flow is used with spiral wound membranes. Each geometry has advantages and disadvantages. Dead end geometry is generally cheaper to set up and therefore has lower initial capital costs. However, it is very vulnerable to membrane fouling, which reduces the effectiveness of the membrane. This is usually the geometry set up for small scale lab experiments. The tangential flow devices are more cost and labor-intensive, but they are less susceptible to fouling due to the sweeping effects and high shear rates of the passing flow. Most commercial industrial membrane separations are done using spiral wound cross flow membrane modules. | |||
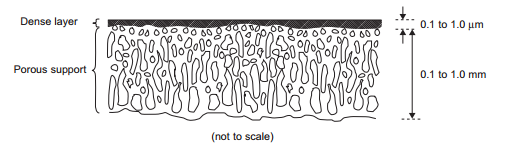
The first big breakthrough for membrane technology was the creation of the asymmetric membrane. In this type of membrane, there is a very thin dense polymer portion of the membrane that is cross linked with less dense, spongier, support polymer. The dense part of the membrane does most of the filtering while the spongy section provides structural support ensuring the membrane will not break. This allows the dense portion to be thinner which increases overall flux through the membrane. The early asymmetric membranes were made with all the same polymer. State of the art membranes are called thin film composite membranes. They are essentially asymmetric membranes were the dense thin portion is made from a different polymer than the spongy support. This enables the best support polymer to be paired with the best filtering polymer allowing for much more effective membranes. Current thin film composite membranes are usually made with a polyamide thin film layer supported by polysulfone. | |||
[[File:Asymmetric_membrane.PNG|frame|Figure 22. Asymmetric membrane structure]] | |||
===Designing a Membrane System=== | |||
When designing a filtration system you must first specify your permeate (product) flow rate and the flux of your membrane along with the membrane area of your selected unit. Number of units can be found using the following equation | |||
<math>N_{E} = \tfrac{Q_{p}}{f * S_{E}} </math> | |||
Where Q_p is the permeate flow rate, f is the flux, and S_E is the area of the selected membrane. | |||
Once you have also specified a recovery, you can choose a number of stages (series membranes as opposed to parallel). Generally if the desired recovery is below 50%, the separation can be done in one stage. Recoveries higher than this are generally best done with 2 stages. More stages can be used to achieve very high recoveries but these are rare. Once you have chosen a number of stages, you can use the following equations to determine the number of membranes in each stage | |||
<math>\begin{align} | |||
R & = {\tfrac{1}{1 - Y}}^{1/n} \qquad & R &= \tfrac{N_{E_{i}}}{N_{E_{i+1}}} | |||
\end{align}</math> | |||
Where Y is the recovery and n is the number of stages and R is the staging ratio. N_E_i is the number of membrane elements in stage i. | |||
===Applications=== | |||
====Food Industry==== | |||
Due to the fact that MD can be conducted at relatively low feed temperatures, it was successfully tested in many areas where high temperature applications lead to degradation of the process fluids especially in food processing. It was demonstrated that MD can be used for the concentration of milk, for the recovery of volatile aroma compounds from black currant juice, and for the concentration of many other types of juices including orange juice, mandarin juice, apple juice, sugarcane juice, etc. | |||
====Reverse Osmosis==== | |||
Reverse osmosis is the most widely used membrane separation process. In this process, fresh water passes through the membrane while dissolved salts and other solids are rejected and stay in the concentrate. In this process, feed water is pressurized in order to overcome the osmotic potential difference between the salty retentate and the fresh water desired. These processes are generally run using spiral wound membrane cylinders using a cross flow setup. | |||
===Membrane Model=== | |||
The two most important components when considering different membranes are the permeability, which will determine flux through the membrane, and selectivity, which will determine what passes through the membrane and how much. The flux through a membrane is defined as: | |||
<math>M_i = \tfrac{P_i}{\delta}(p_{\text{i,f}} - p_{\text{i,p}})</math> | |||
Where <math>M_i</math> is the molar flux of component i, Pi is the permeability of the membrane for component i, δ is the membrane thickness, and <math>p_(i,f)</math> and <math>p_(i,p) </math> are the partial pressures of component i on the feed side and permeate side respectively. | |||
The average flux across a long cylindrical membrane such as the spiral wound module is given by: | |||
<math> \int_0^Lm \frac{M_i,dx}{L_m}</math> | |||
Where Lm is the length of the cylinder and x is length in meters | |||
Membrane selectivity of the ideal separation factor is given as the ratio of the permeability of one substance over another as shown: | |||
<math> S_{ij} = \tfrac{P_i}{P_j} </math> | |||
where <math>S_{ij} </math> is the selectivity of the membrane for component i over j. | |||
==Cyclones== | |||
Centrifugal Separators, more commonly know as Cyclones, are one of the most widely used gas-solid separators. They are typically used for particles that are 5μm or larger, but if agglomeration occurs they can sometimes separate particles as small as particles as small as .5μm. These cyclones can be designed for high efficiency separations or for high throughput (Towler, 2013). | |||
[[File:cyclone.png|thumb|center|Figure 23. Reverse-flow Cyclone (Towler, 2013)|500x300px]] | |||
Cyclones are popular in industry because of their low capital costs, low maintenance costs, low pressure drop, temperature and pressure limitations are only caused the materials, they work as dry equipment, and they don't take up much space (EPA). | |||
They are not without their disadvantages. They have low collection efficiencies for small particles, they are unable to handle sticky materials and high efficiency units often have large pressure drops (EPA). | |||
===Design=== | |||
Design Procedure | |||
There are multiple design methods for cyclones. The deign method discussed below is the Stairmand method. This involved creating two designs one of which was for high throughput and the other which was designed for high efficiency. Using these base designs future cyclones can simply be estimated using scaling factors (Towler, 2013). | |||
1. Select whether efficiency or performance is more important for your design | |||
2. Determine the particle size distribution in your stream | |||
3. Estimate the number of cyclones needed | |||
3. Calculate the cyclone diameter | |||
4. Calculate the scale up factor | |||
5. Calculate the cyclone performance and overall efficiency | |||
6. Calculate the cyclone pressure drop | |||
7. Cost the system | |||
====Particle Size==== | |||
The diameter of particles separated by cyclones are governed by this design equation. This equation is dependent upon the standards of the cyclone and how much you are trying to deviate from its standard operating conditions. Looking into the equation it shows that a larger internal cyclone diameter, higher flowrate, higher difference in density, and lower viscosity result in smaller particles being seperated. The whole term after d<sub>1</sub> is known as a scale up factor. When designing a cyclone you would determine the flow rate, viscosity, particle size, and density difference would be known prior to using this design equation, so this would be used to optimize the diameter of the cyclone you were designing (Towler, 2012). | |||
[[File:Towler 10.8.PNG|center|Figure 24. Reverse-flow Cyclone (Towler, 2013)|500x300px]] | |||
d<sub>1</sub>=mean diameter of particle separated at the standard conditions, at the chosen | |||
separating efficiency | |||
d<sub>2</sub>= mean diameter of the particle separated in the proposed design, at the same | |||
separating efficiency | |||
D<sub>c1</sub> =diameter of the standard cyclone | |||
D<sub>c2</sub> =diameter of proposed cyclone | |||
Q<sub>1</sub> =standard flow rate | |||
Q<sub>2</sub> =proposed flow rate | |||
Δρ<sub>1</sub> =solid-fluid density difference in standard conditions | |||
Δρ<sub>2</sub> =density difference, proposed design | |||
μ <sub>1</sub>=test fluid viscosity | |||
μ <sub>2</sub>=viscosity, proposed fluid. | |||
[[File:Towler10.44.PNG||thumb|center|Figure 25. (a) Standard Cyclone Dimensions (b) High Gas Rate Cyclone (Towler, 2013)|500x300px]] | |||
====Efficiency==== | |||
Efficiency is the percentage of total solids that are removed from the stream The graphs below were experimentally for the two standard designs Stairmand Method. This graph has the efficiency vs the particle size (Towler, 2012). | |||
[[File:Towler10.45.PNG||thumb|center|Figure 26. Performance curves, standard conditions. (a) High-efficiency cyclone performance curves, standard conditions. (b) High gas-rate cyclone (Towler, 2013)|600x400px]] | |||
If you have to scale the efficiency it can be estimated by the point where a horizontal line from the efficiency of the original particle size intersects the new particle size. A demonstration is on the graph below. | |||
[[File:Towler 10.46.PNG||thumb|center|Figure 27. Scaled Performance Curve(Towler, 2013)|500x300px]] | |||
====Pressure Drop==== | |||
One of the most important design factors in a cyclone is its pressure drop. The pressure drop occurs because entry and exit losses, kinetic energy losses, and friction losses that occur inside the cyclone. The pressure drop can be calculated using the following equation (Towler, 2013). | |||
[[File:Towler 10.9.PNG|center|Figure 28. Reverse-flow Cyclone (Towler, 2013)|500x300px]] | |||
The variables are defined as follows: | |||
DP = cyclone pressure drop, millibars; | |||
rf = gas density, kg/m3 | |||
; | |||
u<sub>1</sub> = inlet duct velocity | |||
u<sub>2</sub> = exit duct velocity | |||
r<sub>t</sub> = radius of circle to which the center line of the inlet is tangential | |||
r<sub>e</sub> = radius of exit pipe | |||
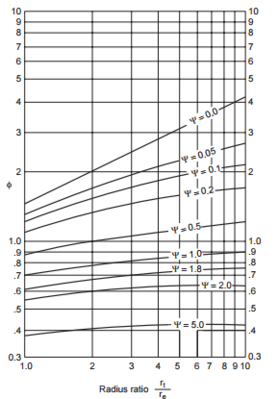
φ =factor from figure below | |||
ψ = fc(A<sub>s</sub>/A<sub>1</sub>) | |||
f<sub>c</sub> = friction factor | |||
A<sub>s</sub> = surface area of cyclone exposed to the spinning fluid | |||
A<sub>1</sub> = area of inlet duct | |||
[[File:Towler_10.47.PNG|thumb|center|Figure 29. Cyclone Pressure Drop Factor (Towler, 2013)|600x400px]] | |||
====Cost Estimation==== | |||
The following values are represented in 2002 dollars. Cost is mostly a function of the amount of gas that need to be passed through the cyclone and the units are in standard meters cubed per second (sm<sup>3</sup>/sec). | |||
The costs can be broken down into three categories: | |||
Capital Costs: $4600-$7600 sm<sup>3</sup>/sec | |||
Operations and Maintenance: $1500-$18,000 sm<sup>3</sup>/sec | |||
Annualized Cost: $2800-$29000 sm<sup>3</sup>/sec | |||
This all comes to $.47-$440 per metric ton of pollutant removed. The high variation in these costs have to do with smaller units being much less efficient per unit of pollutant removed. Furthermore the cost is also effected by the amount of particulates that are in the gas to begin with. For example a gas with 10 g/sm<sup>3</sup> and air with 100sm g/sm<sup>3</sup> would have similar pumping costs, but very different cost per unit of solids removed (EPA). | |||
==Other Separation Processes== | ==Other Separation Processes== | ||
===Extraction=== | ===Extraction=== | ||
Liquid-liquid extraction is a process for components with overlapping boiling points and azeotropes. The process requires a solvent such that some of the components of the mixture are soluble, and then the components will be separated based on this solubility in the liquid. This process can operate at moderate temperatures and pressures, so is not very energy intensive. However, a distillation column is required to extract the solvent for recycle. More recently, supercritical fluids have replaced liquid solvents in some processes for L/L extraction, due to the solute’s ability to more rapidly diffuse through them. The issue with these fluids, however, is that they must be operated at extremely high pressures and temperatures, increasing both capital and operating expenses of the process | Liquid-liquid extraction is a process for components with overlapping boiling points and azeotropes. The process requires a solvent such that some of the components of the mixture are soluble, and then the components will be separated based on this solubility in the liquid. This process can operate at moderate temperatures and pressures, so is not very energy intensive. However, a distillation column is required to extract the solvent for recycle. More recently, supercritical fluids have replaced liquid solvents in some processes for L/L extraction, due to the solute’s ability to more rapidly diffuse through them. The issue with these fluids, however, is that they must be operated at extremely high pressures and temperatures, increasing both capital and operating expenses of the process (Peters & Timmerhaus, 2003). | ||
===Crystallization=== | ===Crystallization=== | ||
This process recovers solutes that have been dissolved in solution. The resulting product is in the solid phase. Depending on the material properties of the solute and solvent, the solute is recovered by precipitation after cooling, removal of solvent, or adding precipitating agents. Crystallizers are designed based on phase equilibria, solubilities, rates and amounts of nuclei generated, and rates of crystal growth. Every crystallization process is a unique system, so plant evaluation is usually required before complete implementation. Crystallization can be performed in both batch and continuous processes, and design features can control crystal size to an extent | This process recovers solutes that have been dissolved in solution. The resulting product is in the solid phase. Depending on the material properties of the solute and solvent, the solute is recovered by precipitation after cooling, removal of solvent, or adding precipitating agents. Crystallizers are designed based on phase equilibria, solubilities, rates and amounts of nuclei generated, and rates of crystal growth. Every crystallization process is a unique system, so plant evaluation is usually required before complete implementation. Crystallization can be performed in both batch and continuous processes, and design features can control crystal size to an extent (Peters & Timmerhaus, 2003). | ||
===Membrane Separation=== | ===Membrane Separation=== | ||
This separations process uses selectively permeable membranes to separate components in a mixture. Typically, one of the components will freely pass through the barrier while the other components will not. The stream that passes through the membrane is the permeate and the stream that does not pass is the retentate. The driving force behind this separation is a pressure gradient. Membrane separation is beneficial because it can separate mixtures at the molecular and small particle level. Furthermore, there is no phase change required so the energy input is low. Limitations of this process include achieving high product purity, incompatibility with certain stream components, low operating temperature, and low flow rates. Although membrane separation is generally not scaled up, examples of scaled-up membrane separation include seawater desalination and hydrogen recovery | This separations process uses selectively permeable membranes to separate components in a mixture. Typically, one of the components will freely pass through the barrier while the other components will not. The stream that passes through the membrane is the permeate and the stream that does not pass is the retentate. The driving force behind this separation is a pressure gradient. Membrane separation is beneficial because it can separate mixtures at the molecular and small particle level. Furthermore, there is no phase change required so the energy input is low. Limitations of this process include achieving high product purity, incompatibility with certain stream components, low operating temperature, and low flow rates. Although membrane separation is generally not scaled up, examples of scaled-up membrane separation include seawater desalination and hydrogen recovery (Peters & Timmerhaus, 2003). | ||
===Adsorption=== | ===Adsorption=== | ||
Adsorption involves an adsorbent and adsorbate. The adsorbent is typically a solid, and will typically separate the adsorbate from the stream. This process usually includes a desorption step that regenerates the adsorbent for further use. Raising the temperature or increasing the concentration of the adsorbate can reverse the adsorption process. Although the recycle of the adsorbent is a very economic design feature, the downside of this step is that it results in a cyclic process, which introduces complexity to the overall process. Industrial applications of this process are for bulk separations and gas purification. The adsorption/desorption process in these situations involves a large amount of heat transfer, which design engineers must take into account when sizing and selecting equipment material | Adsorption involves an adsorbent and adsorbate. The adsorbent is typically a solid, and will typically separate the adsorbate from the stream. This process usually includes a desorption step that regenerates the adsorbent for further use. Raising the temperature or increasing the concentration of the adsorbate can reverse the adsorption process. Although the recycle of the adsorbent is a very economic design feature, the downside of this step is that it results in a cyclic process, which introduces complexity to the overall process. Industrial applications of this process are for bulk separations and gas purification. The adsorption/desorption process in these situations involves a large amount of heat transfer, which design engineers must take into account when sizing and selecting equipment material (Peters & Timmerhaus, 2003). | ||
===External Field/Gradient Separation=== | ===External Field/Gradient Separation=== | ||
These separations use external force fields or temperature gradients to separate responsive molecules or ions. The use of these processes is fairly limited to a few specialized industrial applications | These separations use external force fields or temperature gradients to separate responsive molecules or ions. The use of these processes is fairly limited to a few specialized industrial applications (Peters & Timmerhaus, 2003). | ||
===Settling and Sedimentation=== | ===Settling and Sedimentation=== | ||
In settling processes, solid particles or liquid drops are separated from a stream by gravity. The stream can be in either the liquid or gas phase. For vapor-liquid mixtures, flash drums are generally used to separate the mixture. The velocity of the vapor must be less than the settling velocity of the liquid drops for this separation to occur. For liquid-liquid separation, the horizontal velocity of the fluid must be low enough to allow the low-density droplets to rise to the interface and the high-density droplets to move away from the interface and coalesce. In sedimentation, the result of the process is a more concentrated slurry. Typically a flocculating agent is used to aid in the settling process. One way to perform this separation is to use a cone-shaped tank with a slowly revolving rake that scrapes and moves the thickened slurry to the center of the cone for removal [4 | In settling processes, solid particles or liquid drops are separated from a stream by gravity. The stream can be in either the liquid or gas phase. For vapor-liquid mixtures, flash drums are generally used to separate the mixture. The velocity of the vapor must be less than the settling velocity of the liquid drops for this separation to occur. For liquid-liquid separation, the horizontal velocity of the fluid must be low enough to allow the low-density droplets to rise to the interface and the high-density droplets to move away from the interface and coalesce. In sedimentation, the result of the process is a more concentrated slurry. Typically a flocculating agent is used to aid in the settling process. One way to perform this separation is to use a cone-shaped tank with a slowly revolving rake that scrapes and moves the thickened slurry to the center of the cone for removal (Peters & Timmerhaus, 2003). | ||
[[File:Example.jpg]] | |||
====Clarifiers==== | |||
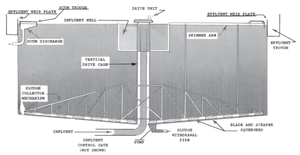
[[File:Circular_Clarifier.png|300px|thumb|bottom|Figure 30: Circular clarifier with some components labelled.]] [[File:Rectangular_Clarifier.png|300px|thumb|bottom|Figure 31: Rectangular clarifier with some components labelled.]] | |||
Clarifiers are one of the methods used for the continuous removal of particulate solids from liquids through sedimentation by gravity. Applications include process water pretreatment, waste water treatment, and drinking water purification. Historically, clarifiers were originally developed to limit nutrient input into surface water due to fear of eutrophication. Today, they have a number of uses, particularly in wastewater treatment processes, metal removal, disinfection, and membrane pretreatment. The process helps removed dissolved solids, silt, and undesirable metals from the water, making it more suitable for downstream processes as well as human consumption (Wilson, 2005). | |||
Clarifiers are typically used in conjunction with coagulation or flocculation agents, which promote dissolved particles to join into clumps and settle out of solution (Towler and Sinnot, 2012). Clarifiers typically consist of a large circular tank with a rotating rake at the base which scrapes settled solids towards the center. In the case of a rectangular clarifier, they are scraped to one side. Diagrams of both are represented in figures 9 and 10, respectively (NMED Surface Water Quality Bureau, 2015). Separated solids are allowed to settle to the bottom of the tank as a sludge, whereupon they are collected by the rake and disposed of properly. In the case of floating contaminants, it is possible for the clarifier to include a skimmer as well. | |||
Clarifier efficiency varies with certain factors, including the settling characteristics of solids removed and the surface overflow rate of the tank. Clarifier efficiency can be found using the following relation: | |||
<math>\begin{align} | |||
E_{TSS} &= E_{TSSmax}\left ( 1 - e^\frac{\lambda}{SOR} \right ) | |||
\end{align}</math> | |||
where <math>E_{TSS}</math> is the efficiency of total suspended solids (TSS) removal, <math>E_{TSSmax}</math> is the maximum possible efficiency, <math>\lambda \left [\frac{m}{d} \right ]</math> is the settling constant, and <math>SOR \left [\frac{m^3}{m^2 d} \right ]</math> is the surface overflow rate. The effect of flocculation chemicals on TSS can be seen in figure 11. However, it should be noted that chemical addition will increase sludge quantity and may have an adverse effect on plant aesthetics, which increases maintenance costs (Wilson, 2005). | |||
[[File:Chem_Addition.png|200px|thumb|bottom|Figure 32: The effect of flocculating agents on total suspended solids removal in clarifiers.]] | |||
=====Lamella Clarifiers===== | |||
Lamella clarifiers use inclined plates in order to maximize the settling area for solids. Solids continue to settle into a hopper at the bottom of the tank while clarified water exits up through the inclined plates. This allows for the design of a smaller tank, which leads to large savings in capital costs. A lamella clarifier is pictured in figure 12. | |||
[[File:Lamella_Clarifier.png|300px|thumb|bottom|Figure 33: A lamella clarifier with components labeled.]] | |||
Typically, inclined plates are installed at an angle of 45 to 60 degrees and spaced 40 to 120 mm apart, which increases effective settling surface area by a factor of 6 to 12 compared to traditional clarifiers. For effective use, it is recommended that the Reynolds number be below 2000, Froude number higher than 10<sup>-5</sup>,and detention time be longer than 3 to 5 minutes. For this implementation, the equations are as follows: | |||
<math>\begin{align} | |||
N_{Re} &= \frac{VR}{\nu} \\ | |||
N_{Fr} &= \frac{V^2}{Rg} | |||
\end{align}</math> | |||
where <math>R</math> refers to the hydraulic radius, which is the cross-sectional area of the lamella, <math>V</math> is the liquid velocity, <math>\nu</math> is the kinematic viscosity, and <math>g</math> is the gravitational constant (Wilson, 2005). | |||
=====Advantages===== | |||
Clarifiers offer a proven, relatively inexpensive solution for solids removal. The chemical coagulants used are cheap and provide a low operating cost as well as simple maintenance. Construction is typically simple, leading to low capital costs and equipment that is easy to accommodate and maintain. Their design is also flexible, with various options such as skimmers and scrapers offering increased removal efficiency (Wilson, 2005). Operation of clarifier tanks also has lower energy requirements than membrane filtration for solids removal, given that most of the separation is aided by gravity. Water exiting clarifier units has a silt density index (SDI) averaging 4.0, which is low enough for further membrane treatment such as reverse osmosis (Prihasto, 2009). | |||
=====Disadvantages===== | |||
Clarifiers necessitate low turbulence to prevent resuspension of solids. This essentially requires a low entrance velocity, which can limit the production rate of certain processes or call for more clarifier units, which would drive up costs. Furthermore, clarifiers require frequent cleaning before sludge becomes too difficult to remove and reduces effectiveness. In the case of lamella clarifiers, sludge buildup on the inclined plates results in uneven flow distribution which could harm efficiency (US EPA, 2003). For this reason, maintenance requirements for lamella clarifiers are higher, but they can be reduced through the implementation of removable plates (Wilson, 2005). Clarifiers also only remove solids, so pH will not be affected, leading to the need for further pH adjustment (NMED Surface Water Quality Bureau, 2015). | |||
=====Clarifier Design Calculations and Typical Design Values===== | |||
======Detention Time====== | |||
Detention time (DT) is the time is takes for a unit of water to travel from the inlet of the clarifier unit to the outlet. During typical operations, the design value for this is 2 to 3 hours. | |||
<math>\begin{align} | |||
DT &= \frac{Tank\ Volume}{Influent\ Rate} | |||
\end{align}</math> | |||
======Surface Overflow Rate====== | |||
Surface overflow rate (SOR) measures the flow into the clarifier per square foot of surface area. Typical design values are 400 to 800 gal/day/sq. ft. | |||
<math>\begin{align} | |||
SOR &= \frac{Volumetric\ Flow\ Rate}{Surface\ Area} | |||
\end{align}</math> | |||
======Weir Overflow Rate====== | |||
Weir overflow rate (WOR) describes the flow in gallons per day per linear foot of weir. Typical values are 10,000 gal/day/ft. | |||
<math>\begin{align} | |||
WOR &= \frac{Volumetric\ Flow\ Rate}{Weir\ Length} | |||
\end{align}</math> | |||
======Solids Loading Rate====== | |||
Solids loading rate (SLR) describes the mass of solids in the clarifier influent per square foot of surface area. This value should not exceed 30 lbs/day/sq. ft. | |||
<math>\begin{align} | |||
SLR &= \frac{Solids\ Mass\ Flow\ Rate}{Surface\ Area} | |||
\end{align}</math> | |||
===Flotation=== | ===Flotation=== | ||
Flotation is a process designed for specific solid-solid mixtures. It works by generating gas bubbles in a liquid that attach to selected solid particle. Afterwards, the particles rise to the liquid surface where they are removed by an overflow weir or mechanical scraper. The separation depends on the surface properties of the particles and its preference to attach to the gas bubbles. To meet the necessary requirements of the flotation process, a number of additives can be used to control things like the pH of the liquid-solid mixture, the activity of the solid surface, and the froth that can assist in separation. The bubbles can be produced by gaseous dispersion, dissolution, or electrolysis of the liquid | Flotation is a process designed for specific solid-solid mixtures. It works by generating gas bubbles in a liquid that attach to selected solid particle. Afterwards, the particles rise to the liquid surface where they are removed by an overflow weir or mechanical scraper. The separation depends on the surface properties of the particles and its preference to attach to the gas bubbles. To meet the necessary requirements of the flotation process, a number of additives can be used to control things like the pH of the liquid-solid mixture, the activity of the solid surface, and the froth that can assist in separation. The bubbles can be produced by gaseous dispersion, dissolution, or electrolysis of the liquid (Peters & Timmerhaus, 2003). | ||
===Centrifugation=== | ===Centrifugation=== | ||
This process is similar to external field separation in that an external force field is applied to separate a mixture. When gravity separation is too slow due to particle densities, particle size, settling velocity, or the formation of an emulsion, centrifugation is commonly used. Centrifugal force increases the total force acting on the particle and results in faster separation times. This process is generally used to separate solids from liquids, however it can also be used to separate two liquids with very different densities | This process is similar to external field separation in that an external force field is applied to separate a mixture. When gravity separation is too slow due to particle densities, particle size, settling velocity, or the formation of an emulsion, centrifugation is commonly used. Centrifugal force increases the total force acting on the particle and results in faster separation times. This process is generally used to separate solids from liquids, however it can also be used to separate two liquids with very different densities (Peters & Timmerhaus, 2003). | ||
===Drying=== | ===Drying=== | ||
Drying is performed to remove liquid from a liquid-solid mixture and produce a dry solid. Water is most often the liquid removed, but organic liquids are removed from solids on occasion as well. The heat required to vaporize the liquid is usually obtained by a series of gas-solid contacting devices. Feed condition and temperature sensitivity of the solid dictate the type of contacting device that is used. There are two groups of dryers that differ by the dependence of either mechanical means or fluid motion for gas solid contact. Another feature of dryers is to use either direct (hot gas) or indirect (conductive surface) heating | Drying is performed to remove liquid from a liquid-solid mixture and produce a dry solid. Water is most often the liquid removed, but organic liquids are removed from solids on occasion as well. The heat required to vaporize the liquid is usually obtained by a series of gas-solid contacting devices. Feed condition and temperature sensitivity of the solid dictate the type of contacting device that is used. There are two groups of dryers that differ by the dependence of either mechanical means or fluid motion for gas solid contact. Another feature of dryers is to use either direct (hot gas) or indirect (conductive surface) heating (Peters & Timmerhaus, 2003). | ||
===Evaporation=== | ===Evaporation=== | ||
Evaporators separate solvents from a solution by evaporation. The difference between evaporation and distillation is that evaporation requires the solute be nonvolatile. Because of this, a high separation can be achieved with one stage. Evaporators are essentially reboilers, so evaporation is a very energy-intensive process with a high thermal economy | Evaporators separate solvents from a solution by evaporation. The difference between evaporation and distillation is that evaporation requires the solute be nonvolatile. Because of this, a high separation can be achieved with one stage. Evaporators are essentially reboilers, so evaporation is a very energy-intensive process with a high thermal economy (Peters & Timmerhaus, 2003). | ||
===Filtration=== | ===Filtration=== | ||
Filtration is a process that separates a mixture of solid in a liquid or gas by passing the mixture through a porous medium in which the particles do not pass. Filtration is done by either cake filtration (particles found on the surface of the filter) or depth filtration (particles found within the filter). Cake filtration is generally performed with a cloth as the filtration medium | Filtration is a process that separates a mixture of solid in a liquid or gas by passing the mixture through a porous medium in which the particles do not pass. Filtration is done by either cake filtration (particles found on the surface of the filter) or depth filtration (particles found within the filter). Cake filtration is generally performed with a cloth as the filtration medium (Peters & Timmerhaus, 2003). | ||
==Conclusion== | ==Conclusion== | ||
| Line 234: | Line 750: | ||
==References== | ==References== | ||
Air Control Technology Fact Sheet. Enviornmental Technology Fact Sheet. http://www3.epa.gov/ttncatc1/dir1/fcyclon.pdf | |||
Belter PA, Cussler EL, Hu WS. Bioseparations: Downstream Processing for BIotechnology. New York: John Wiley; 1998. | |||
Biegler LT, Grossmann IE, Westerberg AW. Systematic Methods of Chemical Process Design. Upper Saddle River: Prentice Hall; 1997. | |||
Danckwerts P (1965) The Absorption of Gases in Liquids. Pure and Applied Chemistry UK 10:625-642. | |||
Development Document for the Final Effluent Limitations Guidelines and Standards for the Metal Products and Machinery Point Source Category (Report). US Environmental Protection Agency. 2003. | |||
Erwin, D. Industrial Chemical Process Design. New York: McGraw Hill, Professional Engineering; 2002. | |||
Hage, David S. 1999Affinity Chromatography: A Review of Clinical Applications. Clinical Chemistry 45(5): 593–615. | |||
Harrison RG, Todd P, Rudge SR, Petrides, DP. Bioseparations Science and Engineering. New York: Oxford University Press; 2003. | |||
Hage, David S. 1999Affinity Chromatography: A Review of Clinical Applications. Clinical Chemistry 45(5): 593–615. | |||
HisPur Cobalt Resin - Thermo Fisher Scientific N.d. https://www.thermofisher.com/order/catalog/product/89964, accessed February 21, 2016. | |||
Lamella Plate Clarifier. Hydro International Web site. Available at: http://www.hydro-int.com/uk/products/lamella-plate-clarifier?s=0&r=uk. Accessed February 2, 2016. | |||
Lean Oil Absorption. PetroGas Systems Web site. Available at: http://petrogassystems.com/technology/natural-gas-processing-and-dew-point-control/lean-oil-absorption. Accessed February 19, 2014. | |||
Lichty, Jordan J., Joshua L. Malecki, Heather D. Agnew, Daniel J. Michelson-Horowitz, and Song Tan 2005Comparison of Affinity Tags for Protein Purification. Protein Expression and Purification 41(1): 98–105. | |||
Merichem Gas Technologies. ®LO-CAT PROCESS available at http://www.merichem.com/images/casestudies/Desulfurization.pdf Accessed 6 Feb. 2015. | |||
Miller L.N. & Zawacki T.S. , US 4080424, "Process for acid gas removal from gaseous mixtures", issued 21 Mar 1978, assigned to Institute of Gas Technology | |||
NMED Surface Water Quality Bureau, New Mexico Water Systems Operator Certification Study Manual, New Mexico Environment Department, 2015. | |||
Peters MS, Timmerhaus KD. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw Hill; 2003. | |||
Prihasto, N; Lui, Q; Kim, S. Pre-treatment strategies for seawater desalination by reverse osmosis system. 2009; 249(1): 308-316. doi:10.1016/j.desal.2008.09.010 | |||
Schmidt Eberhard (2012) Waste Gases, Separation and Purification. Ullman’s Encyclopedia of Industrial Chemistry Germany 2:174-181. | |||
Seider, W.D., Seader, J.D., and Lewin, D.R. (2004). ''Process Design Principles: Synthesis, Analysis, and Evaluation.'' New York: Wiley. | |||
Stripping Column. Alfa Laval Web site. Available at: http://www.alfalaval.com/solution-finder/products/soft-column/Documents/Stripping%20Column.pdf. Accessed February 19, 2014. | |||
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013. | |||
Turton, R.T., Bailie, R.C., Whiting, W.B., and Shaewitz, J.A. (2003). ''Analysis, Synthesis, and Design of Chemical Processes'' Upper Saddle River: Prentice-Hall. | |||
Wankat, P.C. (2012). ''Separation Process Engineering.'' Upper Saddle River: Prentice-Hall. | |||
Wilson, T.E., Clarifier Design, 2nd Ed., McGraw-Hill: New York, 2005. | |||
Latest revision as of 05:58, 23 February 2016
Authors: Nick Pinkerton, [2014] Karen Schmidt, [2014] James Xamplas, [2014] Emm Fulk, [2015] and Erik Zuehlke, [2015] John Dombrowski [2016] , Brett Sleyster [2016] , Robert Cignoni [2016] , and Osman Jamil [2016]
Stewards: David Chen, Jian Gong, and Fengqi You
Date Presented: February 9, 2014 /Date Revised: February 1, 2014
Introduction
Essentially all chemical processes require the presence of a separation stage. Most chemical plants comprise of a reactor surrounded by many separators. Separators have a countless number of jobs inside of a chemical plant. A separator can process raw materials prior to the reaction, remove incondensable gases, remove undesired side products, purify a product stream, recycle materials back into the process, and many other jobs that are essential to the process.
Chemical engineers must understand the science of separation and the variety of ways that separation can take place. There are many ways to perform a separation some of these including: distillation, absorption, stripping, and extraction. The science of separation revolves around the presence of two phases that are in contact and equilibrium (Wankat, 2012).
Theory
Vapor-Liquid Equilibrium
Separation processes are based on the theory of vapor-liquid equilibrium. This theory states that streams leaving a stage in a separation process are in equilibrium with one another. The idea of equilibrium revolves around the idea that when there is vapor and liquid in contact with one another they are in constantly vaporizing and condensing. Different components in the mixture will condense and vaporize at different rates. There are three types of equilibrium conditions that can be subdivided into thermal, mechanical and chemical potential categories. These separate equilibrium states are given as:
Distillation
Flash Distillation
Flash Distillation is one of the simpler separation processes to be employed in a chemical plant. The main premise of flash distillation is that a portion of a liquid feed stream vaporizes in a flash chamber or a vapor feed condenses. Vapor-liquid equilibrium will cause the vapor phase and the liquid phase to have different compositions. The more volatile component of the mixture will compose of a larger portion of the vapor. This simple separation is easy to manufacture but does not result in large degrees of separation.
Flash distillation requires a feed stream that is pressurized and heated and then passed through a valve into a flash drum. The large pressure drop across the valve will result in a partial vaporization of the fluid. Vapor will be removed overhead from the flash drum while the remaining liquid will collect at the bottom of the drum and be removed. Most flash drums will contain an entrainment eliminator which is a screen that prevents liquid from being carried into the vapor effluent. Figure 2 shows a simple overview of the flash distillation process. As shown, there is a heater that flows into a let-down valve where the two-phase flow begins. Variables y and x are the mole fractions of the more volatile component in the vapor and liquid effluents, respectively.
Column Distillation
Distillation columns are the most widely used separation technique used in the chemical industry, accounting for approximately 90% of all separations (Wankat, 2012). Distillations in columns consist of multiple trays that each act at their own equilibrium conditions. Large columns are able to perform complete separations of binary mixtures as well as more complex multi-component mixtures.
Stages
Columns are separated into stages by the presence of trays. These trays allow for vapor-liquid contact and equilibrium to occur. Typically, the more stages in a column, the larger separation that can be achieved. There are many different types of trays that can be used in a column.
Sieve Trays
The simplest and least expensive tray type is the sieve tray which is a sheet of metal with holes punched into it to allow vapor flow. Sieve trays can have different hole patterns and sizes that will affect the tray efficiency and flow rates.
Sieve Tray Design Procedure
The design of these plates is done through a trial-and-error process. Most commercial process simulations (such as HYSYS) have default tray designs, and automatically specify dimensions. However, these dimensions selected or calculated by the simulations may not give the best performance for your system, so it is valuable to understand how to design the sieve trays and how specific parameters may affect performance. Hand calculations using the following methods can be used to guide the simulation programs to better design. This section will use sample data to work through an example of the process. The following is a general list of steps for designing a sieve plate:
1. Calculate the maximum and minimum vapor and liquid flow rates for the turndown ratio required.
This data can be collected from a McCabe-Thiele diagram and/or from process simulation data.
Data from McCabe Thiele diagram, for example:
Number of stages = 10
Slope of top operating line = 0.185
Slope of bottom operating line = 1.43
Top composition = 98.8 mol% acetone
Bottom composition = 4 mol% acetone (a)
Minimum reflux ratio = 0.31
Collect data rom mass balances:
MW of feed = (0.6 kmol a/kmol)*(58 kg/kmol a) + (0.4 kmol acetic acid/kmol)*(60 kg/kmol aa) = 59 kg/kmol
Feed rate = 100 kmol/h
Using material balances... D = 57.3 kmol/h ; B = 42.7 kmol/h
Vapor rate, V = D(1+R) = 57.3 kmol/h (1+.31) = 75.1 kmol/h
Below feed line, slope of BOL = 1.43 = L'm/V'm (liquid rate/vapor rate)
-- Also, V'm = L'm - B (mass balance on bottom of column)
-- V'm= 99.3 kmol/h and L'm = 142.0 kmol/h
2. Collect or estimate the system physical properties.
Here it is important to know information about both the top and bottom of the column. Useful information includes temperature, pressure, column pressure drop (a common assumption is 100 mmH2O per plate), densities, molecular weights, surface tensions, and number of stages (which can be estimated from the McCabe-Thiele diagram).
Conditions at the top of the column:
T = 59oC
P = 760 mmHg = 101,333 N/m2 (atmospheric pressure)
Estimate the properties of the top as those of the vapor (acetone here), which is typically the only thing present in binary distillation.
Surface tension = 18.9 x 10-3 N/m
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_v}
= 2.55 kg/m3
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_L}
= 790 kg/m3
MW = 58 kg/kmol
Conditions at bottom of the column:
T = 104oC
Pressure -- There is a pressure drop in the column, from the bottoms to the top. A common assumption is a pressure drop per plate of 100 mmH2O
Change in P = (9.81 x 103 Pa/mmH2O)(100 mmH2O)(1000 kgH2O/m3)(16 plates) = 15,696 N/m2
Pbottoms = Patm + Pgauge = 101,333 N/m2 + 15,696 N/m2 = 117,030 N/m2
Estimate the properties of the bottoms as those of the heavy component (acetic acid here):
Surface tension = 13.9 x 10-3 N/m
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_v}
= 2.68 kg/m3
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_L}
= 1049 kg/m3
MW = 60 kg/kmol
3. Select a Trial Plate Spacing
The plate spacing will depend on the column diameter and operating conditions. Plate spacings from 0.15 m to 1.0 m are typically used. The smaller the diameter, the smaller the spacing. Small columns will use close spacing. Columns with diameters above 1.0 m, plate spacings of 0.3 m to 0.6 m are normally used. A good initial estimate is 0.5 m.
4. Estimate the column diameter, based on flooding considerations.
Vapor and liquid flow rates will vary along the column, so plate design needs to be considered both above and below the feed. Using plate spacing and FLV, you can obtain the value of K from Figure 4.
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle F_{\text{LV}} = (slope_{\text{operating line}})*\sqrt{\frac{\rho_v}{\rho_L}}}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle F_{\text{LV(bottom)}} = 0.072}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle F_{\text{LV(top)}} = 0.011}
Thus, K1_bottom = 0.90 and K1_top = 0.85
Next, correct those values of K1 for surface tensions:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle K_1' = K_1*(\frac{\sigma}{\sigma_{\text{water}}})^{\text{0.2}}}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \sigma = {\text{surface tension}} }
Next, calculate uf, the vapor velocity through the net area at flooding:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle u_f = K_1\sqrt{\frac{\rho_L - \rho_V}{\rho_V}}}
There is a range of vapor and liquid flow rates in which the column needs to be operated. Too low or too high of rates can result in various inefficiencies in the column operation, as shown in Figure 5. For example, if the vapor rate is too high, flooding will occur. However, it is not safe to operate on the flooding line. Instead, columns are typically designed for 80% of flooding at the maximum flow rate.
Obtain a new velocity with this 80%, and use the velocity to calculate a a maximum volumetric flowrate. Using this and the velocity, we can calculate a net area necessary for vapor flow through the plate. Also need to assume a downcomer area. Now a column corss sectional area can be calculated.
5. Decide the liquid flow arrangement.
Common flow arrangements are single pass (cross flow), double pass, and reverse flow. Using conditions at the bottom of the column, calculate the max volumetric flow rate. Use this flow rate and the column diameter to determine the preferred flow arrangement from the chart below.
6. Make a trial plate layout: downcomer area, active area, hole area, hole size, weir height.
Standard sizes for trays -- and good assumptions for the first iteration -- are: weir height, hw = 50mm ; hole diameter, Dh = 5mm ; plate thickness, tpl = 5mm. From the graph below, the ratio of downcomer area (Ad) to column cross-sectional area (Ac) can be determined from the ratio of weir length (lw) to column diameter (Dc) and vice versa.
7. Check the weeping rate
Compare the actual vapor velocity to the minimum vapor velocity -- if velocity is too low fluid will "weep" through the tray holes. If the weeping rate is unsatisfactory, return to step 6 and choose different values for the plate layout dimensions. From the chart in step 4, it can be seen that there is a minimum vapor flow rate below which the liquid "weeps" from the tray above.
For the remaining steps in this design process, it is recommended to check your assumptions after each step and revise them as necessary in order to maintain operation in the "sweet spot" of the vapor rate vs. liquid rate plot. Additional iterations may be required as you move through the procedure.
Calculate the maximum liquid flow rate. Calculate the minimum liquid flow rate at 70% turndown (recommended). Calculate the height over the weir as Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle h_o=750[\frac{L_w}{p_Ll_w}]^\frac{2}{3}}
8. Check the plate pressure drop
If the pressure drop calculated here is too high, return to step 6.
Proceed to step 9 if the pressure drop assumption is valid.
9. Check the downcomer backup.
If the downcomer backup is too high, return to step 6 or 3.
The plate spacing affects the amount of fluid in the downcomer. Calculate the level in the downcomer and the residence time of the fluid to see if the values are valid. Note that residence times greater than 3 seconds are acceptable.
Proceed to step 10 if residence time is acceptable.
10. Decide plate layout details.
Determine calming zones, the unperforated areas at the inlet and outlet sides of the plate. The width of each zone is usually made the same. Recommended values are: below 1.5 m diameter, 75 mm; above, 100 mm. The unperforated area can be calculated from plate geometry. Also check the hole pitch, or the distance between hole centers. It should not be less than 2.0 hole diameters. A normal range is between 2.5 and 4.0 hole diameters. The shape must also be specified. Square and equilateral triangle holes are used.
11. Recalculate the percentage flooding based on the chosen column diameter.
An assumption of 80% flooding was chosen so that operation would occur in the "sweet spot." This assumption must be checked by calculating the flooding percentage for a given column diameter. uv = (max volumetric flow rate)/(net area)
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle %flooding = \frac{u_v}{u_f}}
If the hole pitch is unsatisfactory, return to step 6.
12. Check entrainment
If too high, return to step 4 Use the graph below to determine entrainment from FLV.
The value for fractional entrainment can be used to re-estimate the column efficiency, and reevaluate the number of trays needed. Can return to step 1 for more accurate estimates.
13. Optimize design.
After returning to step 1 to reevaluate the number of trays, it is valuable to repeat steps 2 through 12 to find the smallest diameter and plate spacing acceptable at the lowest cost.
14. Finalize the design.
Optional: draw up the plate specification and sketch the layout of the plate.
Bubble-Cap Trays
Bubble-cap trays consist of a weir around each hole in the tray which is covered with a cap that has holes or slots to allow vapor passage. Entrainment is about three times larger than a sieve tray. Bubble-cap trays require larger tray spacing than sieve tray design. Bubble-cap trays have been known to have problems with coking, polymer formation, or high fouling mixtures. Recently, very few new bubble-cap columns are being built due to the expense and marginal benefits. However, engineers will likely encounter bubble-cap columns still currently in operation.
Flow Patterns
Cross flow columns are the most common pattern for distillation columns. For liquid flows between 50 and 500 Gal/min, a cross flow column is appropriate. When liquid flow is increased above 500 Gal/min, an engineer should consider designing a double pass or multi-pass column. This will reduce the liquid gradient on the tray and reduce the downcomer loading (Wankat, 2012).
Column Sizing
Column height will be dependent on the amount of trays required and the spacing between the trays. Normally, tray spacing of 0.15 m to 1 m is used. For columns, above 1 meter in diameter, 0.5 m can be used as an initial estimate.
Column diameter is influenced by the vapor flow rate in the column. The trays can not have excess liquid entrainment or high pressure drops; therefore, vapor velocity in the column must be maintained at a reasonable level.
An equation based on the Souders and Brown equation can be used as an estimate for the max allowable superficial vapor velocity,
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \hat u_v = (-0.171l_t^2 + 0.27l_t - 0.047){\frac{\rho_L - \rho_v}{\rho_v}}^{1/2}}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle l_t} is the plate spacing in meters, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_L} is the density of the liquid stream, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_V} is the density of the vapor stream.
Column diameter, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle D_c} , can then be estimated using the relation,
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle D_c = \sqrt{\frac{4\hat{V_w}}{\pi\rho_v\hat{u_v}}}}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \hat{V_w}} is the maximum vapor rate in kg/s (Towler et al., 2013).
Distillation Applications
Distillation is a process that can be implemented in various scales. There is both laboratory scaled distillation as well as very large industrial distillation. Other applications for distillation include food/alcohol processing and herb distillation for the perfume and medical industries. Typically laboratory scaled distillation occurs in batches whereas industrial distillation (e.g. fractional distillation of crude oil) occurs continuous with a constant distillate and bottom effluent streams.
Some applications of distillation are concerned the top stream only, some the bottom stream only and others both streams can be used for future products. In alcohol distillation for example, the water that is separated from the ethanol/water binary solution is discarded as waste water. In fractional distillation of crude oils, the heavy hydrocarbons at the bottom of the column are collected and sold along with the light hydrocarbons that appear in higher side draws (Wankat, 2012).
Example Case: Ideal Distillation
Assume an equimolar mixture flowing at 10 mol/s of 20 mol% n-pentane, 30 mol% n-hexane, and 50 mol% n-heptane. Separate the mixture into 3 products: 99% pure n-pentane, 99% pure n-hexane, 99% n-heptane. Assume the feed and products are all liquids at the bubble points. There are two process alternatives to consider in this example. The direct sequence removes the most volatile species, pentane, in the first column, and then separates hexane and heptane in the second column. The indirect sequence separates the heaviest product, heptane, and then separates pentane from hexane in the second column. This example will consider the direct sequence. Next, we must decide if these species exhibit fairly ideal behavior during distillation. Since the n-alkanes have very similar properties, it is safe to assume they will display close to ideal behavior. The next step is to look up the boiling points of the 3 species. In this case, the normal boiling points of pentane, hexane, and heptane are 309 K, 342 K, and 372 K, respectively. Also, it is a good idea to look up relative volatilites, to further verify near-ideality of the mixture, but also to obtain the information necessary for the Underwood method, which we will employ to obtain a solution. The next step is to write out material balances based on molar flows and the design specifications. They go as follows:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_I(nC5) + \mu_{II}(nC5) = 2 mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_I(nC6) + \mu_{II}(nC6) + \mu_{III}(nC6) = 3 mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{II}(nC7) + \mu_{III}(nC7) = 5 mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_I(nC5) = 99\mu_I(nC6)}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{II}(nC5) = (5/990)\mu_{II}(nC6)}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{II}(nC7) = (5/990)\mu_{II}(nC6)}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{III}(nC7) = 99\mu_{III}(nC7)}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu} represents the molar flow, and the subscript represents the product stream.
Solving this system of equations:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_I(nC5) = 1.985\ mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{II}(nC5) = 0.015\ mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_I(nC6) = 0.020\ mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{II}(nC6) = 2.930\ mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{III}(nC6) = 0.050\ mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{II}(nC7) = 0.015\ mol/s}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_{III}(nC7) = 4.985\ mol/s}
At this point we have enough information to use Underwood's method to estimate the minimum vapor flows in the column. The following three equations are used in Underwood's method:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \sum_i \frac{\alpha_{ik}}{\alpha_{ik}-\phi}f_i = (1-q)F}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle (R_{min}+1)D = \sum_i \frac{\alpha_{ik}}{\alpha_{ik}-\phi}d_i = V_{min}}
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \bar R_{min}B = -\sum_i \frac{\alpha_{ik}}{\alpha_{ik}-\phi}b_i = \bar V_{min}}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \alpha_{ik}} is the relative volatility of species Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle i} to species Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle k} , Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle f_i} the molar flow of species Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle i} in the feed, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle q} the fraction of the feed that joins the liquid stream at the feed tray, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle F} the total molar flow of the feed, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle D} the molar flow of the distillate, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle R_{min}} the minimum reflux ratio Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle (=L_{min}/D)} , Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle d_i} the molar flow of species Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle i} in the distillate, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V_{min}} the minimum vapor flow possible in the top section of the column to accomplish the desired separation, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \bar R_{min}} the minimum reboil ratio Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle (=\bar V_{min}/B)} , Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle b_i} the molar flow of species Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle i} in the bottoms product, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \bar V_{min}} the minimum vapor flow in the bottom section of the column. The final variable, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \phi} , will be solved for using the first Underwood equation, and it's value will be decided based on the relative volatilities of the key components in the column.
So, after solving the first Underwood equation, we get two values for Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \phi} , 3.806 and 1.462. Because 3.806 is between the relative volatilities of the key components, we will substitute that value for Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \phi} into the second Underwood equation. Doing so for both columns gives Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V_{min} = 6.4\ mol/s} for the first column and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V_{min} = 8.9\ mol/s} for the second column, for a total minimum vapor flow of 15.3 mol/s. The process would then be repeated for the indirect sequence, and the decision for which process to use would be justified by the process with the overall minimum vapor flow (Biegler et al., 1997).
Absorption
Description of Absorption
Another separation process used in industry is absorption, which is used to remove a solute from a gas stream. It accomplishes this by contacting the gas mixture with a liquid solvent that readily absorbs the undesirable components from the gas stream, purifying the gas stream. This separation process is determined by the inputs of the liquid flow rate, temperature, and pressure.
The absorption factor, which can be determined mathematically, determines how readily a component will absorb in the liquid phase. The absorption factor of component i is
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle A_i=L/K_iV}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle L} is the liquid flow rate entering the column, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V} is the vapor flow rate entering the column, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle K_i} is the vapor/liquid equilibrium ratio for component i (Peters & Timmerhaus, 2003). Higher absorption factors result in higher absorptivity into the liquid and a decrease in the number of trays required for separation, however a diminishing return occurs after the absorption factor is greater than 2.0. An absorption factor of 1.4 is most commonly used.
In general absorption can be seperated into two overarching categories, physical and chemical absorption. In physical absorption, the unwanted solute in the gas is absorbed into the liquid phase because solubility of the component is higher in the liquid phase than the gas phase. In chemical absorption the solute is removed from the gas via a reaction with the solvent, this reacted product is then transported into the liquid phase (Danckwerts 1965). There are two types of chemical absorption reversible and irreversible. Generally reversible chemical absorption is preferred as the solvent can be put through a stripper and regenerated so it can be recycled back to the absorption process (Wankat, 2012).
Absorption Apparatus
There are five major apparatus used for absorption in industrial application. These five pieces of equipment are spray absorbers (or towers), ejector (venturi) scrubbers, packed columns, trayed columns, and film absorbers (Schmidt, 2012).
Spray Tower vs Ejector Scrubber
In both spray tower and the ejector scrubber nozzles are employed to produce small solvent droplets. These small droplets increase the surface area of the liquid to gas contact allowing for the maximum amount of mass transfer to occur between the gas mixture and the liquid. The major difference between the two nozzle equipment designs is the configuration and type of nozzles. In the ejector scrubber shown in Figure 11 there is a single nozzle that is generally a higher pressure spray nozzle that produces finer solvent drops allowing for an even greater amount of mass transfer enabling better physical absorption (Schmidt, 2012).
Spray towers on the other hand generally have many nozzle at different heights where the liquid solvent will be sprayed out of to contact the gas running through the tower. This design is used in order to ensure the gas contacts the liquid as throughout the tower. These nozzles are lower pressure than a ejector scrubbers nozzle and thus physical mixing is worse in this configuration. Since physical mixing is generally worse in this configuration it is usually used in conjunction with a chemical absorption process. The other major difference between the ejector scrubber and the spray tower is that gas and liquid flow is cocurrent in the former while it is countercurrent in a spray tower. A spray tower absorber is shown below in Figure 12 (Schmidt, 2012).
Tower Type Absorption Apparatus
Packed column absorbers and tray column absorbers have very high efficiencies for the removal of an unwanted solute in the gas stream. The major disadvantage a trayed column has when compared to a packed column is the pressure drop. The pressure drop in a packed column is generally very low, whereas in between each tray of a trayed column pressure drop can be quite large. However the advantages inherent to trayed columns become clear when one needs the solvent to have a high concentration of the component to be removed from the gas stream. This is most important in the case where there is a very low concentration of the component in the gas stream and the specification states the solvent must contain a high concentration of that component. In this case the flow rate of the solvent may not be high enough for a packed column, however in a trayed column the solvent flow rate can be near zero for operation (Schmidt, 2012). Packed and trayed column internals are very similar to the setups found in the respective distillation columns.
For a trayed column the plate efficiency can be calculated using O'Connell's Correlation which invovles the Henry's Law constant, total system pressure, and solvent viscosity at the operating temperature (Towler & Sinnott, 2013).
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle x=0.062*\frac{\rho_s*P}{\mu_s*H*M_s}}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle x} is the tray efficiency, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_s} is the density of the solvent in Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle kg/m^3} , Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle P} is the total pressure of the system in Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle N/m^2} , Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu_s} is the solvent's viscosity in Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle mNs/m^2} , Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle H} is the Henry Law constant in Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 1/(Nm^2*(mol fraction))} , and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle M_s} is the molecular weight of the solvent.
A packed towers height can be determined using the equations below when concentration of solute is below 10% so that the assumption that the flow of gas and liquid will be essentially constant throughout the column holds (Towler & Sinnott, 2013). The height of packing Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Z} is given by the following equation:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Z=\frac{L_m}{K_G*a*P}*\int\limits_{y_2}^{y_1} \frac{dy}{y-y_e}\,}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle P} is the total pressure, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle a} is the interfacial surface area per unit volume, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle y_1} and are the mol fractions of the solute in the gas stream at the bottom and top of the column respectively, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle G_m} is the molar gas flow rate per unit cross-sectional area, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle y_e} is the mole fraction of solute in the gas that would be in equilibrium with the liquid concentration.
The first half of the equation before the integral can be called the height of an overall gas-phase transfer unit Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle H_G} and the second part of the equation is the number of overall gas-phase transfer units or Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle N_G} . Using these definitions the above equation can be simplified to
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Z=H_G*N_G}
These equations assist in sizing an absorption column (Towler & Sinnott, 2013).
Film Absorber
The final absorber the film absorber is generally used in the case where the heat of absorption must be removed. The film absorber operates by sending the gas and solvent through a heat exchanger where the solvent creates a thin film on the walls of the tubes and the gas flows through the interior allowing for solute transfer. The good heat transfer present in a film absorber makes it preferable for situations where low temperatures are required for a high recovery of the solute (Schmidt 2012).
Industrial Absorption Processes
An industrial example is lean oil absorption, which is used to separate nitrogen and other impurities from natural gas. A lean oil is contacted with low quality natural gas, and the methane is selectively absorbed by the lean oil, leaving the impurities behind. The methane is subsequently regenerated from the rich oil as high quality natural gas (Petrogas Systems, 2014).
Other common industrial practices of absorption come from sour gas treatment. Amine gas treating is used to remove hydrogen sulfide or carbon dioxide from gas streams via a reversible chemical absorption. In amine gas treating the sour gas is fed to the bottom an absorber where amine solution is fed to the top along with any necessary make up water. The sour gas components are absorbed into the amine via a chemical absorption method. Sweet gas leaves the top of the absorber whereas the amine out of the bottom, now rich with acidic components is sent to a regenerator where the acid gas components are stripped and the acid gas is generally sent to a flare whereas the amine now lean again is recycled back into the first absorber (Miller & Zawacki, 1978). Figure 13 below shows the typical setup of an amine plant. Another type of sour gas treatment that uses absorption is Merichems LO-CAT process which uses a chelated iron to remove hydrogen sulfide from feed gas in the absorption column (Merichem 2015).
Stripping
This process separates solutes from solvents (often after absorption, to purify the solvent so that it can be recycled to an absorber). Stripping will depend on the vapor and liquid flow rates, as well as the temperature and pressure of the column. There is a temperature drop down the column, so columns generally have either an increased operating temperature or decreased operating pressure.
The stripping factor of component i is
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle S_i=K_iV/L}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle K_i} is the vapor/liquid equilibrium ratio, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V} is the vapor flow rate entering the column, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle L} is the liquid flow rate entering the column, will determine how much of solute i will be stripped from the liquid into the vapor phase (Peters & Timmerhaus, 2003). The usual range for the stripping factor is between 1.2 and 2.0, with a stripping factor of 1.4 being most economic.
An example of stripping in industry is the deodorization of food items such as oils. The oil is heated and allowed to trickle down the column while steam flows up from the bottom of the column. At the vapor-liquid interface, volatile components of the oil transfer to the steam and are carried off the top of the column, leaving a purified oil product (Alfa Laval, 2014).
Bioseparations
Importance
As our ability to manipulate and engineer biological systems improves, biological products are becoming an increasingly important source of therapeutics and fuels. The production of fuels from biomass via either the enzymatic breakdown of a feedstock or the secretion of usable lipids from algae is a promising new energy source. Additionally, enzymes, antibodies and other therapeutic proteins have been applied to the treatment of a wide range of diseases. Although each process requires its own set of separations, all follow the same basic format: separation of biomass, product isolation, and product purification (Belter et al., 1998). This section will provide examples of unit operations in each step. Ultimately, the choice of separation process and unit operations will depend on the specific process and product. The descriptions below are examples of the most common bioseparation operations within the general platform (Harrison et al., 2003).
Bioprocesses begin with fermentations or growth operations. In biofuel production processes, this may involve growing algae or breaking down corn or cellulosic biomass. For the production of therapeutics, mammalian or bacterial cells may be grown in a fermentor and the product secreted into the supernatant or harvested from the cells.
Biomass Separations
After fermentation and product production, the solid biomass must first be separated from the desired product. If the product is secreted from the cells, this can be done immediately after fermentation ends. If the product is not secreted, the cells must first be lysed. Cell lysis is the process of lysing, or breaking, the cell in open. Mechanical lysis is the simplest, and involves physically breaking the cell either by mashing (think mortar and pestle) or blending the cells into a homogenous solution in a homogenizer. Chemical lysis is another method, achieved by introducing an osmotic shock or chemically degrading the cell membrane. Additional separation can be achieved by flocculation, which is the process of aggregating biomaterial by charge neutralization or bridging. These larger complexes are easier to separate from smaller molecules (Harrison et al., 2003).
The next step is removing the unwanted biomass from the product in solution. Separation by centrifugation or sedimentation are the most common, although filtration is sometimes also used for processes where a biomass cake is desired. Both methods utilize density differences to separate the product from the solid biomass (Towler and Sinnott, 2013).
Sedimentation
Sedimentation relies purely on the force of gravity, while centrifugation speeds the settling process by subjecting the cells to a centrifugal force. Sedimentation in a settling tank is the simplest method of solid-liquid bioseparation. In this process, biomass in a tank is simply allowed to settle to the bottom over time. While this process is inexpensive, requires little energy and can separate out large volumes of biomass, it generally requires long time periods and is only mostly in very large-scale processes where active centrifugation is difficult (Belter et al., 1998).
Centrifugation
Centrifuges are widely utilized across many processes, and thus a wide variety of scales and designs have been developed. Disk-stack centrifuges, in which the solid phase is deposited onto “shelves” in the center of the spinner and liquid phase is pushed to the outside, are some of the most commonly used centrifuges in industry. They are especially suited to biomass separation processes because they can be built on a large scale and are ideal for separating fine solids from liquids.
Tubular bowl centrifuges are also common and can reach separation efficiencies of up to 90%. Heavier products accumulate along the sides of the bowl, while the light phase flows out the top. They separate products by can be used both to separate solids from liquids and immiscible liquids, such as and oil product and an aqueous broth (Tolwer and Sinnott, 2013).
Centrifugation scale-up is made easier by sigma analysis, which allows for the estimation of appropriate feed rates for different size centrifuges. The sigma factor is dependent on the inner and outer radius of the centrifuge, the angular velocity, and the sedimentation velocity of the solid particles being separated. It can be thought of as the characteristic cross-sectional area with units of [length]2. The sedimentation velocity can be calculated by
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle v_g={\frac{2a^2(\rho-\rho_0)}{9\mu}}g}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle v_g} is the sedimentation velocity, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle a} is the cell or biomass particle diameter, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho} is the particle density, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \rho_0} is the fluid density, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu} is the fluid viscosity. The volumetric flow Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Q} can be estimated by
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Q=(v_g)(\Sigma)} .
The equality
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle {\frac{\Sigma_1}{\Sigma_2}}={\frac{Q_1}{Q_2}}}
can be an easy way to estimate equivalent flow rates between a small-scale centrifuge 1 and larger centrifuge 2 (Harrison et al., 2003).
Example: Centrifugation Scale-up
You are trying to separate a cell of radius 0.4 Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu} m with a density of 1.05 g/cm3 from broth of mostly water (density of 1 g/cm3 and viscosity of 0.01 g/cm s). The sigma factor of the centrifuge you are using is 1 x 106 cm2. A] What volumetric flow rate should you use? B] If you want to scale up the process to a centrifuge with Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \Sigma} = 3 x 106 cm2, what flow rate would you use in the larger centrifuge?
Solution: A] Using the equation for Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle v_g} , and being mindful of units, the sedimentation velocity equals 1.74 x 106 cm/s. The flow rate, then, equals
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Q=(1.74 x 10^-6)(1,000,000) = 1.74 cm^3/s = 0.104 L/min} .
B] Keeping in mind that for the same process, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle v_g1 = v_g2,} and rearranging the sigma factor equality, the new flow rate is
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle Q_2 = {\frac{\Sigma_2 x Q_1}{\Sigma_1}} = {\frac{(3 x 10^6)(0.104)}{1 x 10^6}} = 0.313 L/min }
Product Isolation
Liquid-liquid separation, to extract the product from the aqueous phase, is much less straightforward than liquid-solid extraction. Many methods - especially adsorption, filtration, and precipitation - are similar in principle to operations found in other, non-biological separations. The exact separations used depend on the nature of the product and the scale of the process. These processes are nearly identical to their non-biological counterparts, and their description is left to other sections.
Particular care needs to be taken with protein products because of their instability, and the selection of an appropriate solvent or adsorbent is crucial to a successful process (Harrison et al., 2003).
Product Purification
The final steps of protein purification and polishing remove any remaining contaminants and bring the concentration of product to an appropriate value for applications. Purification processes for food-grade and medical products can be extensive, as sterility and high purity are essential. Purification in fuel-producing processes may be less extensive, depending on the process. Chromatography and crystallization are two common steps in purification and are especially used in industrial scale protein production. Several different types of chromatography exist with the ability to carry out different types of separations. Chromatography is similar to adsorption in that it relies on differences in affinity between solutes and a solid surface. A solution is eluted through a column containing a solid resin with various affinities for the substances in solution. In adsorption, the solutes are evenly saturated throughout the column. Chromatography differs in that solutes are deposited a resin phase before the column is flushed with an elution solvent specific that results in solutes eluted in bands.
Ion Exchange Chromatography
There are two main types of ion exchange columns—anion and cation. Anion exchange resins have a positive charge and are used to retain products with a negative charge. Cation exchange resins have a negative charge and are used to retain products with a positive charge. The pH of the elution buffer is change to force a specific solute to wash out, depending on whether the pH of the buffer is above or below the isoelectric point of the solute (Belter et al., 1998). This is especially useful for the separation of protein product (including antibodies), nucleic acids, and other charged molecules. When the solutes have sufficiently different isoelectric points, the pH of the buffer is manipulated to affect the solute charge and force the product to elute while the solute remains preferentially bound to the resin, or vice versa (Harrison et al., 2003). In general, the most strongly charged molecules will remain in the column for a longer period of time. Elution washes through the weakly bound ions before the more strongly bound ions. Different speeds of elution can be visualized as in figure 16.
Size Exclusion Chromatography
In gel filtration chromatography, small molecules are "trapped' by the porous resin and take longer to flow through the column. Larger products will elute first because the smaller molecules are better able to penetrate the resin. This forces them to take a much longer path through the column, which means it takes longer for them to elute. This operation is often used when there is a distinct difference in size between the desired product and other solutes.
Affinity Separations
Affinity chromatography is very similar to ion exchange chromatography in that the interactions between the material in the column and the molecules in the feed. The main difference is that affinity chromatography can rely on a great variety of types of interactions. Two very common types of affinity are exploited in affinity chromatography columns. The first is immunoaffinity. Proteins are specifically bound by antibodies which can be incorporated onto beads and used in chromatography. Antibodies are designed to bind only a single protein, so these interactions are considered to be highly specific. The protein can be eluted using a buffer that changes the pH or salinity in the column, which adversely affects binding (Hage, 1999).
Proteins can be separated from very complex and unrefined mixtures using this method because of the great specificity. Antibody-protein interactions are so specific that individual proteins can be isolated from blood samples as shown in the figure below. The main drawback of this method is simply that specific interactions between proteins and binding targets do not always exist. Antibodies exist for many proteins, but for others they must be customized or created using some sort of evolution process. This is not a trivial task and can require a significant capital investment (Hage, 1999).
The other main type of affinity chromatography is based on protein specific tags and the molecules or surfaces to which they bind. One of the most common types of protein tags used is the polyhistidine tag. This tag consists of 6-8 consecutive histidine residues which can be added to the exterior of the desired protein product. The addition of this tag requires alterations to the coding sequence of the protein. The polyhistidine tag binds strongly with nickel and cobalt ions. The product with the tag can then be eluted with imidazole—a small molecule with the same structure as the functional group of the amino acid histidine. Imidazole will bind the cobalt and nickel ions more strongly than the histidine in the tag. Along with chromatography, protein tag interactions can be leveraged with the use of beads that can be deposited directly into the solution containing the protein of interest (Lichty et al., 2005).
Different proteins can be purified in this manner with varying levels of efficiency. There is a very high dependence on the size of the protein, electronic properties, and steric considerations. The figure below demonstrates varying separation efficiencies with proteins of different sizes (HisPur Cobalt Resin - Thermo Fisher Scientific, 2016).
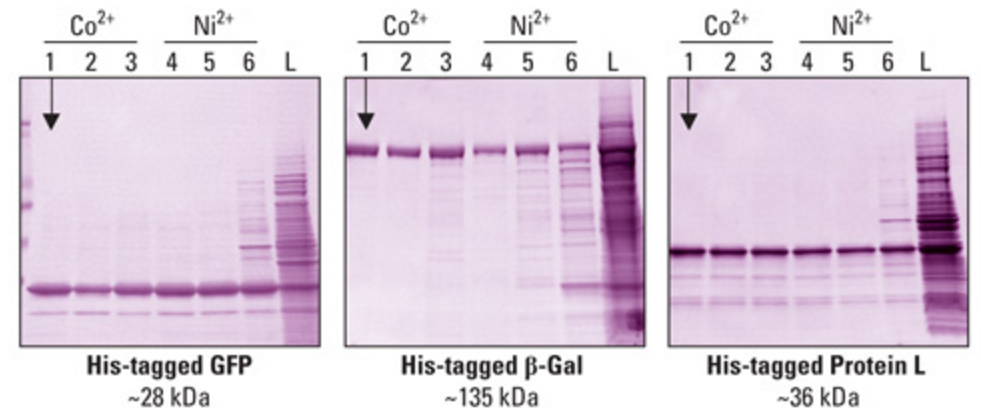
Several other types of tag-bead interactions can be utilized in separations processes. Maltose Binding Protein is a small protein that can be added to a protein of interest. It binds strongly with beads coated in immobilized maltose and can be released by flushing with maltose. As MBP is a full sized protein that typically must be removed from the protein of interest in order for it to be used. In this case, the site specific TEV protease is often used cleave MBP from the protein of interest. In addition, under specific circumstances, other unique tags can be used and provide varying levels of specificity in separations. The Flag tag, 3x Flag tag, Glut tag, and Strep tag. While these are all commonly used, the polyhistidine tag is the most popular because it gives the highest level of specificity. Some of the main purification tags are compared in the table below (Lichty et al., 2005).
| Tag | Size (aa) | Resin | Eluting Agent | Capacity (mg/ml) | Cost/10 mg |
|---|---|---|---|---|---|
| His | 6 | Talon | Imidazole | 5-14 | $18 |
| Maltose Binding Protein | 396 | Amylose | Maltose | 3 | $12 |
| Glutathione S-Transferase | 218 | GSH-Sepharose | Glutathione | 10 | $36 |
| Strep II | 8 | Strep-Tactin Sepharose | Desthiobiotin | 0.1 | $293 |
| FLAG | 8 | Anti-FLAG M2 MAb Agarose | Flag peptide | 0.6 | $1045 |
Unfortunately, based on the contents of the solution, the characteristics of the protein, and the specific product being used, there are extremely large amounts of variation in yield and purity for each type of tag. Therefore, it is crucial to allocate time towards preliminary tests and scale-up experiments with the exact solution from which the protein of interest is being separated. The his tag is typically the most used because, as seen in the table above, it has one of the lowest costs and highest yields. In addition, because it is only a 6 amino acids, it often is not cleaved from the protein of interest which minimizes the number of steps required in the overall purification process (Lichty et al., 2005).
Crystallization
Crystallization, or the formation of solute crystals from a solution, is especially useful in biomolecule separations because it is possible to obtain a 99.9%+ product purity. In crystallization, a diluent is added to the homogeneous solution that reduces the solubility of the product to the point that it “falls out” of solution and crystallizes. It is similar to precipitation but results in the formation of crystals rather than unordered aggregates. Crystallization can be used on a laboratory scale for determining protein structure, on on the industrial scale for antibody and therapeutic protein productions. Batch crystallizers are often used in industry because of their simplicity and inexpensiveness compared to continuous crystallization (Harrison et al., 2003).
Membrane Separation
Membrane separation takes advantage of the selective permeability of membranes; they allow certain particles to pass through and selectively stop other, generally unwanted, particles. The component that passes through is called the permeate and the component stream that is rejected is called the retentate or concentrate. The applicability of membranes comes from the fact that their selectivity is determined by their pore size, which can be controlled during the creation of the membranes. Additionally, Membrane processes do not require heat meaning they generally require less energy than conventional separations technology such as distillation and crystallization. Membrane separations processes are generally classified as microfiltration, ultrafiltration, or nanofiltration depending on the size of the particles to be filtered out.
Membrane Selection, Construction, and Flow Geometries
Membrane permeability and selectivity are the two most important factors to consider when selecting a membrane. For gas separations, the permeation of the gas is usually facilitated by the gas dissolving in the membrane on one side and then evaporating on the permeate side. Therefore permeability depend largely on the solubility of components in the membrane.
The two most commonly utilized membrane configurations are hollow fiber and spiral wound. Hollow fiber is generally the most commonly utilized module for gas separations. These are formed by gluing the two ends of the hollow fiber to a resin forming a closure. The fibers are housed in a shell much like a heat exchanger. The feed flows past thousands of tubes with the permeate flowing into the hollow tubes and out the closure. The retentate then flows out of the shell not having gotten through the membrane.
Spiral wound membranes are created by sealing two membrane sheets back to back on three edges to form a sort of pocket. This fourth open edge is then attached to a porous tube which allows permeate to go through it. Several membrane pockets are attached to a single tube and wrapped around in a spiral.
Flow geometry is usually either dead-end geometry or cross flow geometry. In dead end, the fluid flow is normal to the membrane surface while cross flow is parallel to the membrane surface. Dead end geometry is usually used with hollow fiber membranes while cross flow is used with spiral wound membranes. Each geometry has advantages and disadvantages. Dead end geometry is generally cheaper to set up and therefore has lower initial capital costs. However, it is very vulnerable to membrane fouling, which reduces the effectiveness of the membrane. This is usually the geometry set up for small scale lab experiments. The tangential flow devices are more cost and labor-intensive, but they are less susceptible to fouling due to the sweeping effects and high shear rates of the passing flow. Most commercial industrial membrane separations are done using spiral wound cross flow membrane modules.
The first big breakthrough for membrane technology was the creation of the asymmetric membrane. In this type of membrane, there is a very thin dense polymer portion of the membrane that is cross linked with less dense, spongier, support polymer. The dense part of the membrane does most of the filtering while the spongy section provides structural support ensuring the membrane will not break. This allows the dense portion to be thinner which increases overall flux through the membrane. The early asymmetric membranes were made with all the same polymer. State of the art membranes are called thin film composite membranes. They are essentially asymmetric membranes were the dense thin portion is made from a different polymer than the spongy support. This enables the best support polymer to be paired with the best filtering polymer allowing for much more effective membranes. Current thin film composite membranes are usually made with a polyamide thin film layer supported by polysulfone.
Designing a Membrane System
When designing a filtration system you must first specify your permeate (product) flow rate and the flux of your membrane along with the membrane area of your selected unit. Number of units can be found using the following equation
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle N_{E} = \tfrac{Q_{p}}{f * S_{E}} }
Where Q_p is the permeate flow rate, f is the flux, and S_E is the area of the selected membrane.
Once you have also specified a recovery, you can choose a number of stages (series membranes as opposed to parallel). Generally if the desired recovery is below 50%, the separation can be done in one stage. Recoveries higher than this are generally best done with 2 stages. More stages can be used to achieve very high recoveries but these are rare. Once you have chosen a number of stages, you can use the following equations to determine the number of membranes in each stage
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \begin{align} R & = {\tfrac{1}{1 - Y}}^{1/n} \qquad & R &= \tfrac{N_{E_{i}}}{N_{E_{i+1}}} \end{align}}
Where Y is the recovery and n is the number of stages and R is the staging ratio. N_E_i is the number of membrane elements in stage i.
Applications
Food Industry
Due to the fact that MD can be conducted at relatively low feed temperatures, it was successfully tested in many areas where high temperature applications lead to degradation of the process fluids especially in food processing. It was demonstrated that MD can be used for the concentration of milk, for the recovery of volatile aroma compounds from black currant juice, and for the concentration of many other types of juices including orange juice, mandarin juice, apple juice, sugarcane juice, etc.
Reverse Osmosis
Reverse osmosis is the most widely used membrane separation process. In this process, fresh water passes through the membrane while dissolved salts and other solids are rejected and stay in the concentrate. In this process, feed water is pressurized in order to overcome the osmotic potential difference between the salty retentate and the fresh water desired. These processes are generally run using spiral wound membrane cylinders using a cross flow setup.
Membrane Model
The two most important components when considering different membranes are the permeability, which will determine flux through the membrane, and selectivity, which will determine what passes through the membrane and how much. The flux through a membrane is defined as:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle M_i = \tfrac{P_i}{\delta}(p_{\text{i,f}} - p_{\text{i,p}})}
Where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle M_i} is the molar flux of component i, Pi is the permeability of the membrane for component i, δ is the membrane thickness, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p_(i,f)} and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p_(i,p) } are the partial pressures of component i on the feed side and permeate side respectively. The average flux across a long cylindrical membrane such as the spiral wound module is given by:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \int_0^Lm \frac{M_i,dx}{L_m}}
Where Lm is the length of the cylinder and x is length in meters
Membrane selectivity of the ideal separation factor is given as the ratio of the permeability of one substance over another as shown:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle S_{ij} = \tfrac{P_i}{P_j} }
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle S_{ij} } is the selectivity of the membrane for component i over j.
Cyclones
Centrifugal Separators, more commonly know as Cyclones, are one of the most widely used gas-solid separators. They are typically used for particles that are 5μm or larger, but if agglomeration occurs they can sometimes separate particles as small as particles as small as .5μm. These cyclones can be designed for high efficiency separations or for high throughput (Towler, 2013).
Cyclones are popular in industry because of their low capital costs, low maintenance costs, low pressure drop, temperature and pressure limitations are only caused the materials, they work as dry equipment, and they don't take up much space (EPA).
They are not without their disadvantages. They have low collection efficiencies for small particles, they are unable to handle sticky materials and high efficiency units often have large pressure drops (EPA).
Design
Design Procedure There are multiple design methods for cyclones. The deign method discussed below is the Stairmand method. This involved creating two designs one of which was for high throughput and the other which was designed for high efficiency. Using these base designs future cyclones can simply be estimated using scaling factors (Towler, 2013).
1. Select whether efficiency or performance is more important for your design
2. Determine the particle size distribution in your stream
3. Estimate the number of cyclones needed
3. Calculate the cyclone diameter
4. Calculate the scale up factor
5. Calculate the cyclone performance and overall efficiency
6. Calculate the cyclone pressure drop
7. Cost the system
Particle Size
The diameter of particles separated by cyclones are governed by this design equation. This equation is dependent upon the standards of the cyclone and how much you are trying to deviate from its standard operating conditions. Looking into the equation it shows that a larger internal cyclone diameter, higher flowrate, higher difference in density, and lower viscosity result in smaller particles being seperated. The whole term after d1 is known as a scale up factor. When designing a cyclone you would determine the flow rate, viscosity, particle size, and density difference would be known prior to using this design equation, so this would be used to optimize the diameter of the cyclone you were designing (Towler, 2012).
d1=mean diameter of particle separated at the standard conditions, at the chosen
separating efficiency
d2= mean diameter of the particle separated in the proposed design, at the same separating efficiency
Dc1 =diameter of the standard cyclone
Dc2 =diameter of proposed cyclone
Q1 =standard flow rate
Q2 =proposed flow rate
Δρ1 =solid-fluid density difference in standard conditions
Δρ2 =density difference, proposed design
μ 1=test fluid viscosity
μ 2=viscosity, proposed fluid.
Efficiency
Efficiency is the percentage of total solids that are removed from the stream The graphs below were experimentally for the two standard designs Stairmand Method. This graph has the efficiency vs the particle size (Towler, 2012).
If you have to scale the efficiency it can be estimated by the point where a horizontal line from the efficiency of the original particle size intersects the new particle size. A demonstration is on the graph below.
Pressure Drop
One of the most important design factors in a cyclone is its pressure drop. The pressure drop occurs because entry and exit losses, kinetic energy losses, and friction losses that occur inside the cyclone. The pressure drop can be calculated using the following equation (Towler, 2013).
The variables are defined as follows: DP = cyclone pressure drop, millibars; rf = gas density, kg/m3
u1 = inlet duct velocity
u2 = exit duct velocity
rt = radius of circle to which the center line of the inlet is tangential
re = radius of exit pipe
φ =factor from figure below
ψ = fc(As/A1)
fc = friction factor
As = surface area of cyclone exposed to the spinning fluid
A1 = area of inlet duct
Cost Estimation
The following values are represented in 2002 dollars. Cost is mostly a function of the amount of gas that need to be passed through the cyclone and the units are in standard meters cubed per second (sm3/sec).
The costs can be broken down into three categories:
Capital Costs: $4600-$7600 sm3/sec
Operations and Maintenance: $1500-$18,000 sm3/sec
Annualized Cost: $2800-$29000 sm3/sec
This all comes to $.47-$440 per metric ton of pollutant removed. The high variation in these costs have to do with smaller units being much less efficient per unit of pollutant removed. Furthermore the cost is also effected by the amount of particulates that are in the gas to begin with. For example a gas with 10 g/sm3 and air with 100sm g/sm3 would have similar pumping costs, but very different cost per unit of solids removed (EPA).
Other Separation Processes
Extraction
Liquid-liquid extraction is a process for components with overlapping boiling points and azeotropes. The process requires a solvent such that some of the components of the mixture are soluble, and then the components will be separated based on this solubility in the liquid. This process can operate at moderate temperatures and pressures, so is not very energy intensive. However, a distillation column is required to extract the solvent for recycle. More recently, supercritical fluids have replaced liquid solvents in some processes for L/L extraction, due to the solute’s ability to more rapidly diffuse through them. The issue with these fluids, however, is that they must be operated at extremely high pressures and temperatures, increasing both capital and operating expenses of the process (Peters & Timmerhaus, 2003).
Crystallization
This process recovers solutes that have been dissolved in solution. The resulting product is in the solid phase. Depending on the material properties of the solute and solvent, the solute is recovered by precipitation after cooling, removal of solvent, or adding precipitating agents. Crystallizers are designed based on phase equilibria, solubilities, rates and amounts of nuclei generated, and rates of crystal growth. Every crystallization process is a unique system, so plant evaluation is usually required before complete implementation. Crystallization can be performed in both batch and continuous processes, and design features can control crystal size to an extent (Peters & Timmerhaus, 2003).
Membrane Separation
This separations process uses selectively permeable membranes to separate components in a mixture. Typically, one of the components will freely pass through the barrier while the other components will not. The stream that passes through the membrane is the permeate and the stream that does not pass is the retentate. The driving force behind this separation is a pressure gradient. Membrane separation is beneficial because it can separate mixtures at the molecular and small particle level. Furthermore, there is no phase change required so the energy input is low. Limitations of this process include achieving high product purity, incompatibility with certain stream components, low operating temperature, and low flow rates. Although membrane separation is generally not scaled up, examples of scaled-up membrane separation include seawater desalination and hydrogen recovery (Peters & Timmerhaus, 2003).
Adsorption
Adsorption involves an adsorbent and adsorbate. The adsorbent is typically a solid, and will typically separate the adsorbate from the stream. This process usually includes a desorption step that regenerates the adsorbent for further use. Raising the temperature or increasing the concentration of the adsorbate can reverse the adsorption process. Although the recycle of the adsorbent is a very economic design feature, the downside of this step is that it results in a cyclic process, which introduces complexity to the overall process. Industrial applications of this process are for bulk separations and gas purification. The adsorption/desorption process in these situations involves a large amount of heat transfer, which design engineers must take into account when sizing and selecting equipment material (Peters & Timmerhaus, 2003).
External Field/Gradient Separation
These separations use external force fields or temperature gradients to separate responsive molecules or ions. The use of these processes is fairly limited to a few specialized industrial applications (Peters & Timmerhaus, 2003).
Settling and Sedimentation
In settling processes, solid particles or liquid drops are separated from a stream by gravity. The stream can be in either the liquid or gas phase. For vapor-liquid mixtures, flash drums are generally used to separate the mixture. The velocity of the vapor must be less than the settling velocity of the liquid drops for this separation to occur. For liquid-liquid separation, the horizontal velocity of the fluid must be low enough to allow the low-density droplets to rise to the interface and the high-density droplets to move away from the interface and coalesce. In sedimentation, the result of the process is a more concentrated slurry. Typically a flocculating agent is used to aid in the settling process. One way to perform this separation is to use a cone-shaped tank with a slowly revolving rake that scrapes and moves the thickened slurry to the center of the cone for removal (Peters & Timmerhaus, 2003).
Clarifiers
Clarifiers are one of the methods used for the continuous removal of particulate solids from liquids through sedimentation by gravity. Applications include process water pretreatment, waste water treatment, and drinking water purification. Historically, clarifiers were originally developed to limit nutrient input into surface water due to fear of eutrophication. Today, they have a number of uses, particularly in wastewater treatment processes, metal removal, disinfection, and membrane pretreatment. The process helps removed dissolved solids, silt, and undesirable metals from the water, making it more suitable for downstream processes as well as human consumption (Wilson, 2005).
Clarifiers are typically used in conjunction with coagulation or flocculation agents, which promote dissolved particles to join into clumps and settle out of solution (Towler and Sinnot, 2012). Clarifiers typically consist of a large circular tank with a rotating rake at the base which scrapes settled solids towards the center. In the case of a rectangular clarifier, they are scraped to one side. Diagrams of both are represented in figures 9 and 10, respectively (NMED Surface Water Quality Bureau, 2015). Separated solids are allowed to settle to the bottom of the tank as a sludge, whereupon they are collected by the rake and disposed of properly. In the case of floating contaminants, it is possible for the clarifier to include a skimmer as well.
Clarifier efficiency varies with certain factors, including the settling characteristics of solids removed and the surface overflow rate of the tank. Clarifier efficiency can be found using the following relation:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \begin{align} E_{TSS} &= E_{TSSmax}\left ( 1 - e^\frac{\lambda}{SOR} \right ) \end{align}}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle E_{TSS}} is the efficiency of total suspended solids (TSS) removal, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle E_{TSSmax}} is the maximum possible efficiency, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \lambda \left [\frac{m}{d} \right ]} is the settling constant, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle SOR \left [\frac{m^3}{m^2 d} \right ]} is the surface overflow rate. The effect of flocculation chemicals on TSS can be seen in figure 11. However, it should be noted that chemical addition will increase sludge quantity and may have an adverse effect on plant aesthetics, which increases maintenance costs (Wilson, 2005).
Lamella Clarifiers
Lamella clarifiers use inclined plates in order to maximize the settling area for solids. Solids continue to settle into a hopper at the bottom of the tank while clarified water exits up through the inclined plates. This allows for the design of a smaller tank, which leads to large savings in capital costs. A lamella clarifier is pictured in figure 12.
Typically, inclined plates are installed at an angle of 45 to 60 degrees and spaced 40 to 120 mm apart, which increases effective settling surface area by a factor of 6 to 12 compared to traditional clarifiers. For effective use, it is recommended that the Reynolds number be below 2000, Froude number higher than 10-5,and detention time be longer than 3 to 5 minutes. For this implementation, the equations are as follows:
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \begin{align} N_{Re} &= \frac{VR}{\nu} \\ N_{Fr} &= \frac{V^2}{Rg} \end{align}}
where Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle R} refers to the hydraulic radius, which is the cross-sectional area of the lamella, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle V} is the liquid velocity, Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \nu} is the kinematic viscosity, and Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle g} is the gravitational constant (Wilson, 2005).
Advantages
Clarifiers offer a proven, relatively inexpensive solution for solids removal. The chemical coagulants used are cheap and provide a low operating cost as well as simple maintenance. Construction is typically simple, leading to low capital costs and equipment that is easy to accommodate and maintain. Their design is also flexible, with various options such as skimmers and scrapers offering increased removal efficiency (Wilson, 2005). Operation of clarifier tanks also has lower energy requirements than membrane filtration for solids removal, given that most of the separation is aided by gravity. Water exiting clarifier units has a silt density index (SDI) averaging 4.0, which is low enough for further membrane treatment such as reverse osmosis (Prihasto, 2009).
Disadvantages
Clarifiers necessitate low turbulence to prevent resuspension of solids. This essentially requires a low entrance velocity, which can limit the production rate of certain processes or call for more clarifier units, which would drive up costs. Furthermore, clarifiers require frequent cleaning before sludge becomes too difficult to remove and reduces effectiveness. In the case of lamella clarifiers, sludge buildup on the inclined plates results in uneven flow distribution which could harm efficiency (US EPA, 2003). For this reason, maintenance requirements for lamella clarifiers are higher, but they can be reduced through the implementation of removable plates (Wilson, 2005). Clarifiers also only remove solids, so pH will not be affected, leading to the need for further pH adjustment (NMED Surface Water Quality Bureau, 2015).
Clarifier Design Calculations and Typical Design Values
Detention Time
Detention time (DT) is the time is takes for a unit of water to travel from the inlet of the clarifier unit to the outlet. During typical operations, the design value for this is 2 to 3 hours.
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \begin{align} DT &= \frac{Tank\ Volume}{Influent\ Rate} \end{align}}
Surface Overflow Rate
Surface overflow rate (SOR) measures the flow into the clarifier per square foot of surface area. Typical design values are 400 to 800 gal/day/sq. ft.
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \begin{align} SOR &= \frac{Volumetric\ Flow\ Rate}{Surface\ Area} \end{align}}
Weir Overflow Rate
Weir overflow rate (WOR) describes the flow in gallons per day per linear foot of weir. Typical values are 10,000 gal/day/ft.
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \begin{align} WOR &= \frac{Volumetric\ Flow\ Rate}{Weir\ Length} \end{align}}
Solids Loading Rate
Solids loading rate (SLR) describes the mass of solids in the clarifier influent per square foot of surface area. This value should not exceed 30 lbs/day/sq. ft.
Failed to parse (SVG with PNG fallback (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \begin{align} SLR &= \frac{Solids\ Mass\ Flow\ Rate}{Surface\ Area} \end{align}}
Flotation
Flotation is a process designed for specific solid-solid mixtures. It works by generating gas bubbles in a liquid that attach to selected solid particle. Afterwards, the particles rise to the liquid surface where they are removed by an overflow weir or mechanical scraper. The separation depends on the surface properties of the particles and its preference to attach to the gas bubbles. To meet the necessary requirements of the flotation process, a number of additives can be used to control things like the pH of the liquid-solid mixture, the activity of the solid surface, and the froth that can assist in separation. The bubbles can be produced by gaseous dispersion, dissolution, or electrolysis of the liquid (Peters & Timmerhaus, 2003).
Centrifugation
This process is similar to external field separation in that an external force field is applied to separate a mixture. When gravity separation is too slow due to particle densities, particle size, settling velocity, or the formation of an emulsion, centrifugation is commonly used. Centrifugal force increases the total force acting on the particle and results in faster separation times. This process is generally used to separate solids from liquids, however it can also be used to separate two liquids with very different densities (Peters & Timmerhaus, 2003).
Drying
Drying is performed to remove liquid from a liquid-solid mixture and produce a dry solid. Water is most often the liquid removed, but organic liquids are removed from solids on occasion as well. The heat required to vaporize the liquid is usually obtained by a series of gas-solid contacting devices. Feed condition and temperature sensitivity of the solid dictate the type of contacting device that is used. There are two groups of dryers that differ by the dependence of either mechanical means or fluid motion for gas solid contact. Another feature of dryers is to use either direct (hot gas) or indirect (conductive surface) heating (Peters & Timmerhaus, 2003).
Evaporation
Evaporators separate solvents from a solution by evaporation. The difference between evaporation and distillation is that evaporation requires the solute be nonvolatile. Because of this, a high separation can be achieved with one stage. Evaporators are essentially reboilers, so evaporation is a very energy-intensive process with a high thermal economy (Peters & Timmerhaus, 2003).
Filtration
Filtration is a process that separates a mixture of solid in a liquid or gas by passing the mixture through a porous medium in which the particles do not pass. Filtration is done by either cake filtration (particles found on the surface of the filter) or depth filtration (particles found within the filter). Cake filtration is generally performed with a cloth as the filtration medium (Peters & Timmerhaus, 2003).
Conclusion
Separation is a key part of most chemical processes, and there is a great variety of techniques to perform separation of compounds based on size, volatility, charge, and many other features. A common technique with which the process engineer should be familiar is distillation, but he or she should also be aware of the other available options. Some techniques may be less expensive, less energy-intensive, or more effective than distillation, depending on the specific separation problem. Therefore, the separation strategy should be carefully considered.
References
Air Control Technology Fact Sheet. Enviornmental Technology Fact Sheet. http://www3.epa.gov/ttncatc1/dir1/fcyclon.pdf
Belter PA, Cussler EL, Hu WS. Bioseparations: Downstream Processing for BIotechnology. New York: John Wiley; 1998.
Biegler LT, Grossmann IE, Westerberg AW. Systematic Methods of Chemical Process Design. Upper Saddle River: Prentice Hall; 1997.
Danckwerts P (1965) The Absorption of Gases in Liquids. Pure and Applied Chemistry UK 10:625-642.
Development Document for the Final Effluent Limitations Guidelines and Standards for the Metal Products and Machinery Point Source Category (Report). US Environmental Protection Agency. 2003.
Erwin, D. Industrial Chemical Process Design. New York: McGraw Hill, Professional Engineering; 2002.
Hage, David S. 1999Affinity Chromatography: A Review of Clinical Applications. Clinical Chemistry 45(5): 593–615.
Harrison RG, Todd P, Rudge SR, Petrides, DP. Bioseparations Science and Engineering. New York: Oxford University Press; 2003.
Hage, David S. 1999Affinity Chromatography: A Review of Clinical Applications. Clinical Chemistry 45(5): 593–615.
HisPur Cobalt Resin - Thermo Fisher Scientific N.d. https://www.thermofisher.com/order/catalog/product/89964, accessed February 21, 2016.
Lamella Plate Clarifier. Hydro International Web site. Available at: http://www.hydro-int.com/uk/products/lamella-plate-clarifier?s=0&r=uk. Accessed February 2, 2016.
Lean Oil Absorption. PetroGas Systems Web site. Available at: http://petrogassystems.com/technology/natural-gas-processing-and-dew-point-control/lean-oil-absorption. Accessed February 19, 2014.
Lichty, Jordan J., Joshua L. Malecki, Heather D. Agnew, Daniel J. Michelson-Horowitz, and Song Tan 2005Comparison of Affinity Tags for Protein Purification. Protein Expression and Purification 41(1): 98–105.
Merichem Gas Technologies. ®LO-CAT PROCESS available at http://www.merichem.com/images/casestudies/Desulfurization.pdf Accessed 6 Feb. 2015.
Miller L.N. & Zawacki T.S. , US 4080424, "Process for acid gas removal from gaseous mixtures", issued 21 Mar 1978, assigned to Institute of Gas Technology
NMED Surface Water Quality Bureau, New Mexico Water Systems Operator Certification Study Manual, New Mexico Environment Department, 2015.
Peters MS, Timmerhaus KD. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw Hill; 2003.
Prihasto, N; Lui, Q; Kim, S. Pre-treatment strategies for seawater desalination by reverse osmosis system. 2009; 249(1): 308-316. doi:10.1016/j.desal.2008.09.010
Schmidt Eberhard (2012) Waste Gases, Separation and Purification. Ullman’s Encyclopedia of Industrial Chemistry Germany 2:174-181.
Seider, W.D., Seader, J.D., and Lewin, D.R. (2004). Process Design Principles: Synthesis, Analysis, and Evaluation. New York: Wiley.
Stripping Column. Alfa Laval Web site. Available at: http://www.alfalaval.com/solution-finder/products/soft-column/Documents/Stripping%20Column.pdf. Accessed February 19, 2014.
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.
Turton, R.T., Bailie, R.C., Whiting, W.B., and Shaewitz, J.A. (2003). Analysis, Synthesis, and Design of Chemical Processes Upper Saddle River: Prentice-Hall.
Wankat, P.C. (2012). Separation Process Engineering. Upper Saddle River: Prentice-Hall.
Wilson, T.E., Clarifier Design, 2nd Ed., McGraw-Hill: New York, 2005.