Drop-in Hydrogen Fueling (2014): Difference between revisions
| (2 intermediate revisions by the same user not shown) | |||
| Line 71: | Line 71: | ||
In order to successfully fill an automobile’s fuel tank to full capacity, a minimum tank pressure of 70Mpa is required. Therefore the following equation was solved to determine the capacity of the tank: | In order to successfully fill an automobile’s fuel tank to full capacity, a minimum tank pressure of 70Mpa is required. Therefore the following equation was solved to determine the capacity of the tank: | ||
Where M is the total gravimetric capacity of the storage tank, is the gravimetric density of hydrogen gas at the specified storage conditions of 100MPa and -70˚C, and is the gravimetric storage capacity at 70MPa and -70˚C. The density was calculated using the Peng-Robinson Equation of State | Where M is the total gravimetric capacity of the storage tank, is the gravimetric density of hydrogen gas at the specified storage conditions of 100MPa and -70˚C, and is the gravimetric storage capacity at 70MPa and -70˚C. The density was calculated using the Peng-Robinson Equation of State. M was determined to be 1568.25kg, and the corresponding minimum volume was found to be 25m3. | ||
One safety concern was loss of temperature control of the storage tank, and the resulting over pressurization of the storage tank. To accommodate this, a safety factor in the volume of the tank was designed, so the final volume was 26.5m3. | One safety concern was loss of temperature control of the storage tank, and the resulting over pressurization of the storage tank. To accommodate this, a safety factor in the volume of the tank was designed, so the final volume was 26.5m3. | ||
| Line 275: | Line 275: | ||
The DoE analysis was performed to include estimates for downstream energy costs at the hydrogen fueling station. Their final calculated values can be seen in Table 5, and significant discrepancies between their analysis and our design plan are detailed below. | The DoE analysis was performed to include estimates for downstream energy costs at the hydrogen fueling station. Their final calculated values can be seen in Table 5, and significant discrepancies between their analysis and our design plan are detailed below. | ||
Table 5 | Table 5 (US Department of Energy). Well to Wheels Energy and Greenhouse Gas (GEG) Data | ||
[[File:Table7.PNG]] | [[File:Table7.PNG]] | ||
| Line 328: | Line 328: | ||
==References== | ==References== | ||
Airconco.com. Decibel Scale - Amazing Decibel Infographic [Internet]. London: Airconco (UK) Ltd T/A The Air Conditioning Company [cited 2014 Mar 6]. Available from: http://www.airconco.com/decibel_scale/. | |||
Airproducts.com. News Release: Air Products Introduces Advanced Retail Hydrogen Fuel Dispenser [Internet]. Allentown: Air Products and Chemicals, Inc.; c1996-2015 [cited 2014 Mar 5]. Available from: http://www.airproducts.com/company/news-center/2013/06/0611-air-products-introduces-advanced-retail-hydrogen-fuel-dispenser.aspx. | |||
Alibaba.com [Internet]. Hangzhou: Alibaba.com; c1999-2015 [cited 2014 Mar 12]. Available from: http://www.alibaba.com/showroom/hydrogen-storage-tank.html. | |||
Asq.org. Failure Mode Effects Analysis (FMEA) [Internet]. Milwaukee: American Society for Quality [cited 2014 Mar 7]. Available from: http://asq.org/learn-about-quality/process-analysis-tools/overview/fmea.html. | |||
Circuitbreakerguys.com. Q330H Circuit Breaker by Siemens [Internet]. Burlingame: Circuit Breaker Guys LLC.; c2014 [cited 2014 Mar 7]. Available from: http://www.circuitbreakerguys.com/q330h/?gclid=CM_P2pbJgb0CFbFaMgod_hUAIw. | |||
Couper JR, Penney WR, Fair JR. Rules of Thumb Summary. In: Chemical Process Equipment: Selection and Design. 3rd ed. Boston: Elsevier; 2012. | |||
Cumalioglu I, Ma Y, Ertas A, Maxwell T. High Pressure Hydrogen Storage Tank: A Parametric Study. J Pressure Vessel Technol. 2006 Apr;129(1):216-222. | |||
Detroitmi.gov. Building Codes/Construction Information: Commercial Building Permit/Submittal Checklist [Internet]. Detroit: City of Detroit; c2001-15 [cited 2014 Mar 6]. Available from: http://www.detroitmi.gov/DepartmentsandAgencies/BuildingsSafetyEngineeringEnvironmental/Divisions/LicensesPermits/Permits.aspx. | |||
Gwkent.com. 10 ton Glycol Chiller 3-Phase [Internet]. Ypsilanti: GW Kent; c2010-15 [cited 2014 Mar 12]. Available from: http://www.gwkent.com/10-ton-glycol-chiller-3-phase.html. | |||
Hoodmart.com. Restaurant Fire Suppression Systems: Wet Chemical & Pre-Piped [Internet]. Grafton: HoodMart Inc. [cited 2014 Mar 7]. Available from: http://www.hoodmart.com/restaurant-fire-suppression-system-pfs19.aspx?utm_medium=shoppingengine&utm_source=googlebase&gclid=CIrkm5nHgb0CFeZDMgodq38ASA#.UxpTaF5kLGo. | |||
HyApproval WP2. Handbook for Hydrogen Refueling Station Approval [Internet]. [updated 2008 June 4; cited 2014 Mar 7]. Available from: http://www.hyapproval.org/Publications/The_Handbook/HyApproval_Final_Handbook.pdf. | |||
Hydrogencontest.org. 2011 Contest Residential Fueling with Hydrogen [Internet]. Washington, D.C.: Hydrogen Student Design Contest [cited 2014 Mar 7]. Available from: http://www.hydrogencontest.org/2011.asp. | |||
Hydropac.com. High Pressure Hydrogen Compressors [Internet]. Fairview: Hydro-Pac Inc. [cited 2015 Mar 1]. Available from: http://www.hydropac.com/HTML/hydrogen-compressor.html. | |||
Keytometals.com. Steels for Cryogenic and Low-Temperature Service [Internet]. Zürich: Key to Metals AG; c2015 [cited 2015 Mar 1]. Available from: http://www.keytometals.com/page.aspx?ID=CheckArticle&site=kts&NM=61. | |||
Sae.org. Compressed Hydrogen Surface Vehicle Refueling Connection Devices. [Internet]. Warrendale: SAE International; c2015 [cited 2014 Mar 5]. Available from: http://standards.sae.org/j2600_201211/. | |||
Sakintuna B, Lamari-Darkrim F, Hirscher M. Metal hydride materials for solid hydrogen storage: A review. Int J Hydrogen Energy. 2007 June;32(9):1121-40. | |||
Sandler SI. Chemical, Biological, and Engineering Thermodynamics. 4th ed. Hobeken: John Wiley and Sons; 2006. | |||
Sengpielaudio.com. Damping of sound level (decibel dB) vs. distance [Internet]. London: Airconco (UK) Ltd T/A The Air Conditioning Company [cited 2014 Mar 6]. Available from: http://sengpielaudio.com/calculator-distance.htm. | |||
Sculley J, Yuan D, Zhou H. The current status of hydrogen storage in metal-organic-frameworks-updated. Energy and Environ Sci. 2011 June;4:2721-35. | |||
Siemens.com. Fire Suppression Systems [Internet]. Munich: Siemens AG; c1996-2015a [cited 2014 Mar 7]. Available from: http://w3.usa.siemens.com/buildingtechnologies/us/en/fire-products-and-systems/fire-suppression-system/Pages/fire-suppression-system.aspx. | |||
Siemens.com. Cerberus PRO – An Intelligent Fire Protection System [Internet]. Munich: Siemens AG; c1996-2015b [cited 2014 Mar 7]. Available from: http://w3.usa.siemens.com/buildingtechnologies/us/en/fire-products-and-systems/fire-alarm-systems/cerberus-pro/Pages/cerberus-pro.aspx. | |||
The American Society of Mechanical Engineers. Section VIII. In: Boiler and Pressure Vessel Code. New York: ASME; 2004. | |||
Towler G, Sinnott R. Safety and Loss Prevention. In: Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013a. | |||
Towler G, Sinnott R. Design of Pressure Vessels. In: Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013b. | |||
Trulia.com. 2580 S Schaefer Hwy [Internet]. San Francisco: Trulia, Inc.; c2015 [cited 2014 Mar 7]. Available from: http://www.trulia.com/property/1033919029-2580-S-Schaefer-Hwy-Detroit-MI-48217. | |||
Wastereductionpartners.org. Chillers: Energy Saving Fact Sheet [Internet]. North Carolina Energy Office [cited 2014 Mar 7]. Available from: http://wastereductionpartners.org/phocadownload/userupload/Resources/Energy_Saving_Fact_Sheet_Chillers.pdf. | |||
Wikipedia.org. Hydrogen Embrittlement [Internet]. Wikimedia Foundation [cited 2015 Mar 1]. Available from: http://en.wikipedia.org/wiki/Hydrogen_embrittlement. | |||
US Department of Energy. Module 2 Permitting Hydrogen Motor Fuel Dispensing Facilities. Version 1.0. Pacific Northwest National Laboratory. Report No.: PNNL-14518. 2004 Dec. | |||
US Department of Energy. Centralized Hydrogen Production from Coal Gasification with Sequestration [Internet]. [cited 2014 Mar 6]. Available from: www.hydrogen.energy.gov/docs/cs_central_coal_gasification.doc. | |||
Zoro.com. Noise Control Products [Internet]. Buffalo Grove: Zoro Tools, Inc.; c2011-15 [cited 2014 Mar 5]. Available from: http://www.zorotools.com/g/00060581/k-G2499777?utm_source=google_shopping&utm_medium=cpc&utm_campaign=Google_Shopping_Feed&kw={keyword}&gclid=CPmd_MG9_LwCFaw-MgodQzYAHQ. | |||
Latest revision as of 04:14, 2 March 2015
Authors: Matthew Ardagh, Michael Ashley, Alex Chandel, Eric Jiang, Minwook Kim, Todor Kukushliev, William Lassman (ChE 352 in Winter 2014)
Steward: David Chen, Fengqi You
Date Presented: Winter 2014
Executive Summary
With ongoing energy crisis, constant efforts are made to reach a sustainable energy. Major attention nowadays has been to shift the heavy demand of petroleum fuel to natural gas. Big effort has been made to make such a shift into a reality by making noticeable improvements in the fuel cell vehicle. To facilitate the shift, proper and reliable fueling station for fuel cell vehicle is essential.
The report proposes a design for a portable hydrogen station that can maximize the revenue and therefore facilitate a hydrogen fuel market. The stations can be installed in places where the demand is saturated. Also, when the demand falls low, the portable station can be transferred to different place where the hydrogen demand is higher. The fueling module consists of a compressor, a storage vessel, a dispensing module, and necessary valving systems. The design is kept as minimalistic to minimize the capital cost yet enhance the portability. The site chosen was 2580 S Schaefer Highway in Detroit, Michigan. The site has commodious space of 280x100 ft. and necessary utilities and hookups available to support the designed station along with the car wash and/or oil change center. Despite the portability, the infrastructure will accommodate the state-of-the-art fueling dispensing features.
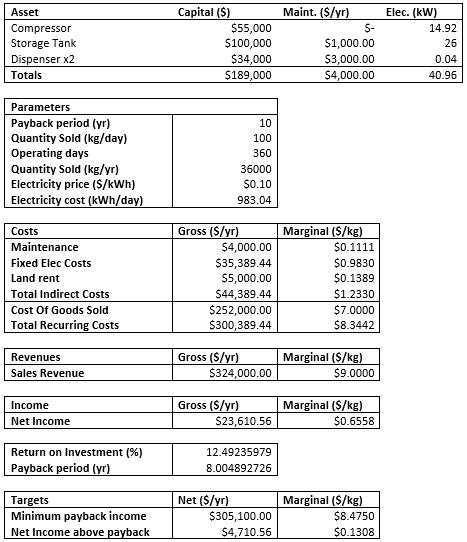
Economic analysis was made without accounting for the tax and restructuring cost. The initial cost of the actuating the design will cost $189,000 in initial capital cost. The sales revenue is estimated to be around $324,000; the annual cost amounts to be $296,355 per year. The income of a single station is $27,645 which will set the payback period to be 8 years.
Introduction
Around the turn of the 21st century, engineering and design were fundamentally changed with the advent of energy and environmental sustainability. With energy prices and harsher standards set by regulating organizations, the modern engineer emphasizes carbon footprint and sustainable design in developing new products and systems. One key technology that is now known to be unsustainable is petroleum, especially as used for automobile fuels. The rising cost of gasoline has motivated the investigation of alternative technologies, such as hydrogen, to be used to power automobiles.
The objective of this study was to design a mobile hydrogen refueling module for high-pressure hydrogen vehicles. The design was required to fulfill the following criteria:
- Dispense 5kg H2 gas in under five minutes
- Refuel vehicles up to 70 MPa
- Support two simultaneous refueling
- Support a 100kg/day demand, plus an additional 48 hours in the event of upstream equipment failure
- Mobile: able to disassemble and reassemble the entire process in under 7 days
- Fit inside a standard ISO container
- Capital investment that is a fraction of 2-4 million USD
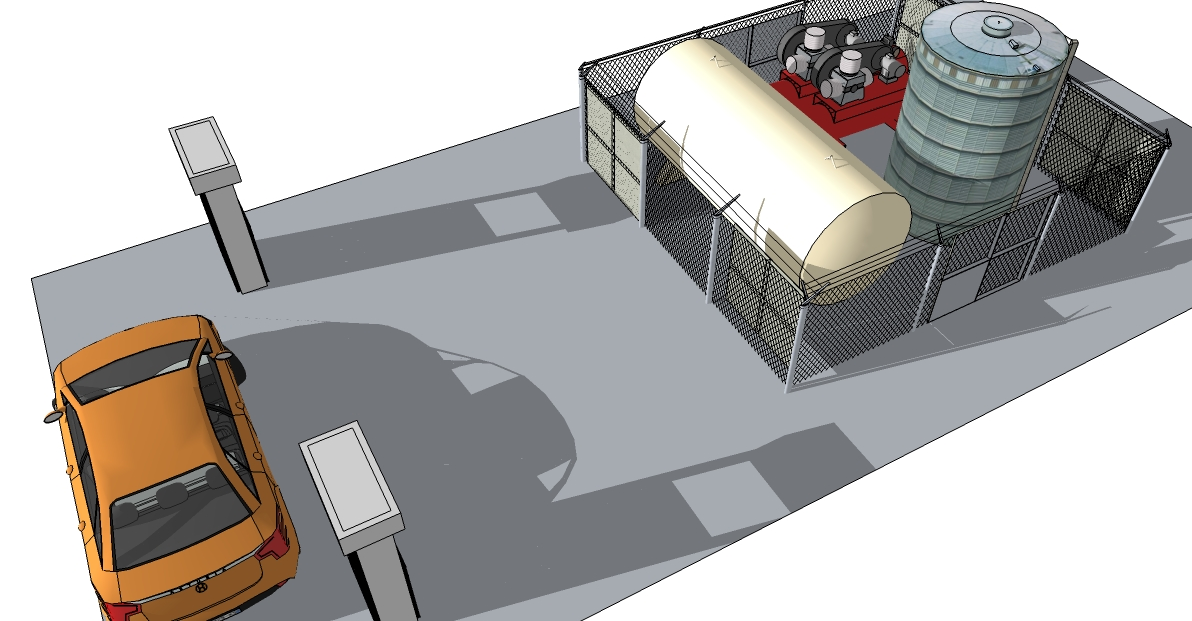
Figure 1 shows a capture of a 3-dimension scale model of the process, in its deployed form.
Process Narrative
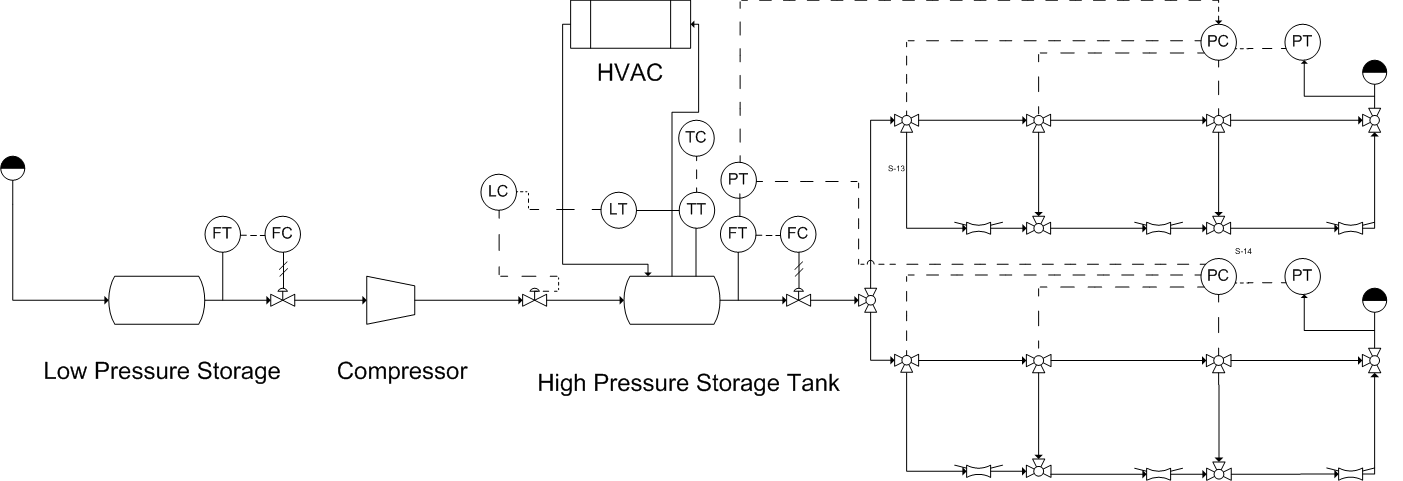
To deliver hydrogen gas to vehicles, the fueling module requires the following equipment: a means to receive delivered hydrogen or capacity for generating it on site, a compressor capable of compressing the hydrogen to the requisite storage conditions, a storage tank, a dispensing system, as well as HVAC and utility equipment and safety equipment (Figure 2).
Figure 2: contains a Piping and Instrumentation Diagram (P&ID) including all major process equipment and safety equipment.
Hydrogen Delivery
We currently recommend buying hydrogen as the main method for procurement of hydrogen by simply purchasing it from existing suppliers at market price. This will avoid capital costs of the upstream production units, which may lower the price of the hydrogen dispensed to clients. Currently, pressurized hydrogen may be purchased at a market price of roughly $7 per kilogram from existing suppliers with the added benefits of requiring fewer stages in the mobile fueling station compressor. In this case, the compressor would only require only two stages to supply hydrogen at the desired dispensing pressure, and so less time to move and construct the station itself. We currently desire to purchase hydrogen gas from a supplier of industrial gas, Praxair. Praxair’s primary method of delivery is by truck, so it will be ideal to choose locations close by Praxair distribution factories and major highways. Our design will allow our storage equipment to directly hook up to the delivery tank of Praxair and allow dispensing to occur.
Two alternatives were also explored for the procurement of hydrogen for the use in the mobile fueling station: steam reforming of methane and water electrolysis. However, these two alternatives are accompanied by significant capital costs, with steam reforming accompanied with complications with the mobility aspect of any ultimate design scheme. Steam reforming of methane utilizes natural gas as a feedstock. Because natural gas is inexpensive, this is the most economically advantageous feedstock for gaseous hydrogen. The hydrogen produced in this manner would then need to be pressurized from atmospheric pressure to the desired dispensing pressures, which would also increase the stages of the compressor chosen to achieve the pressurization. This same problem arises in producing hydrogen through water electrolysis, where hydrogen and oxygen gas are non-spontaneously produced from pure water by a running current. In addition, reforming also runs the disadvantage of being relatively large and immobile. Also, although hydrogen is usually suggested as a sustainable fuel, steam reforming also produces substantial amounts of CO2, creating an irony to “sustainable fuel”. The size and CO2 production inherent in steam reforming discourages its use in our dispensing station.
The electrolyzer requires pure water as a raw material, which will require purification units upstream in the production process. The HG-50 generator offered by HGenerators is capable of producing the specified 200 kg of H2 per day if it is running at full capacity, 24 hours a day. Initial pricing of the HG-50 is estimated at $1.49 million. Operation of this electrolyzer system consumes 215 kWh per hour of operation (costing around $600 per day assuming the average price of electricity in the US at $0.13 per kWh) and 41.667 L of water (costing around $0.40 assuming an average price of electiricty at $1.50 per 1000 gallon in the US). The most favorable selling point of electrolysis is the fact that no carbon dioxide is produced, resulting in more truly clean energy assuming if the source electricity is not dependent on carbon sources. While production of hydrogen would be ideal in our dispensing station due to advantages of sustainability and not requiring additional utilities outside of water and electricity, it is not currently recommended. Due the incredible high capital, which takes up more than half of our intended budget of $2 million, it is not recommended for hydrogen production.
Compressor
To compress the hydrogen gas to the designated storage conditions, 1000 bar, from the delivered pressure of 200 bar, at least 2 stages of compression are required. Hydrogen gas may turn various metal brittle with prolonged exposure, known as hydrogen embrittlement; therefore, the compressor needs to be designed with caution (Towler and Sinnott, 2013a). Because of the low flow rate requirement, a reciprocating compressor may be used (Wikipedia.org). The advantages of the reciprocating compressor are a broad range of pressure ranges, easy maintenance, and relatively cheap price. The downside of the reciprocating compressor is that it generates loud noise.
A suitable compressor that meets above design specification was chosen from the vendor Hydro-Pac (Hydropac.com). A 20-hp compressor that are 94 inches long, 27 inches wide, and 52 inches tall will compress the incoming hydrogen of pressure 200 bar to 1000 bar and send it to the storage. The compressor has the adiabatic efficiency of 35% and generates the noise level of 95 decibels at 1- meter distance, which is under the legal limit. The cost of the compressor was estimated to be 95,000 dollars.
Had the hydrogen was supplied from the electrolysis generation method instead of purchasing directly from the vendor, the compression would require another compressor, intermittent storage, and additional piping systems, which altogether was quoted to be around 250,000 dollars from the same vendor, Hydro-Pac. Both the capital cost and the operating cost for the compressor phase will be higher for the generation method, which validate the decision to purchase the hydrogen directly from the vendor.
Storage
Once the hydrogen is brought to pressure, it passes into the primary storage tank. The storage conditions for the hydrogen gas were selected to be 100 MPa, and -70˚C for the following reasons: 100 MPa ensures that the tank can maintain a sufficient pressure differential to increase the speed at which is filled, and -70˚C ensures that upon expansion, the hydrogen gas is at a temperature range that complies with dispensing guidelines outlined in SAE J2601.
The design specification stated that the refueling module needs to be able to dispense 100kg of H2 fuel per day, with an additional 48 hours supply in event of compressor failure. In the event that the compressor fails before recharging the tank from dispensing its initial 100kg, the tank was therefore designed to have a deliverable capacity of 300kg.
In order to successfully fill an automobile’s fuel tank to full capacity, a minimum tank pressure of 70Mpa is required. Therefore the following equation was solved to determine the capacity of the tank:
In order to successfully fill an automobile’s fuel tank to full capacity, a minimum tank pressure of 70Mpa is required. Therefore the following equation was solved to determine the capacity of the tank: Where M is the total gravimetric capacity of the storage tank, is the gravimetric density of hydrogen gas at the specified storage conditions of 100MPa and -70˚C, and is the gravimetric storage capacity at 70MPa and -70˚C. The density was calculated using the Peng-Robinson Equation of State. M was determined to be 1568.25kg, and the corresponding minimum volume was found to be 25m3.
One safety concern was loss of temperature control of the storage tank, and the resulting over pressurization of the storage tank. To accommodate this, a safety factor in the volume of the tank was designed, so the final volume was 26.5m3.
Common engineering practice is to fix length to diameter ratio of a high pressure storage tank to 1:5, and to use a horizontal storage tank for volumes of this capacity (Sandler, 2006; Couper et al., 2012). The corresponding inside diameter and height of the tank were determined to be 1.9 and 9.4 meters respectively.
The tank was then designed according to ASME BPV Section VIII standards (Towler and Sinnott, 2013b; The American Society of Mechanical Engineers, 2004). The tank will be multilayered with three 5cm layers, manufactured out of A203 stainless steel for low temperature tolerance (Cumalioglu, 2006). The heads of the tank are torispherical, with all welds spot checked. The tank will include a pressure and temperature sensor.
The tank will be coated with a thermo-regulating jacket which will have coolant from the HVAC pass through it as part of the refrigeration loop.
A detailed cost estimate was not obtained due to reluctance of vendors to generate a quote for this complicated tank for a student design competition. However, based on heuristics and comparisons to existing tanks, with some extrapolation, a cost estimate on the order of $100,000 was used in determining the capital investment associated with the storage tank (Keytometals.com; Alibaba.com).
Much of the cost associated with the storage tank is due to the challenging storage conditions of -70 ˚C and 100 MPa. A number of alternatives were considered.
The first alternative was to use a metal hydride to chemisorb the hydrogen gas at low pressure. However, it was determined that metal hydride technologies are not mature enough to meet the storage needs of this project, primarily due to the high heat of adsorption; the adsorbate binds too strongly to the hydrogen and requires heating to drive the gas off when the gas is needed (The American Society of Mechanical Engineers, 2004).
The second alternative considered was to use a Metal Organic Framework (MOF) as an adsorbate. The principal is similar to metal hydrides, but MOFs utilize physisorption instead of chemisorption to bind the hydrogen. For this reason, it suffers from the opposite setback, in that while MOFs can adsorb hydrogen at 77K, they do not perform well at higher temperatures (Sakintuna, 2012).
The main benefit of using an adsorbate is the elimination of the compression stage, which constitutes the largest capital investment and most energy intensive step of preparing hydrogen for fuel conditions. Therefore, these adsorbate technologies make more sense as an onboard storage solution, and as current vehicles do not use these technologies, there is no gain to bypassing the compression stage. Therefore, the final decision to use high-pressure storage was made.
Dispensing System
Hydrogen fuel is dispensed to the consumer’s vehicle from the high-pressure storage tank via a system of three adiabatic expansion valves and three bypass valves. These are required by the J2601 standard, which stipulates that the pressure at the nozzle be no more than 20 MPa above the pressure of the vehicle’s tank. By selective expansion the fuel through either expansion or bypass valves, the hydrogen stream is reduced to a safe pressure for fueling. Additionally, since the storage tank is maintained at -70ºC, the fuel stream will heat up to -40ºC during expansion, providing pre-cooled hydrogen as recommended by J2601 and this contest.
HVAC System
The HVAC system will primarily serve to maintain the extreme cold required to properly store the gaseous hydrogen. This system will use GW Kent’s 10 ton Glycol Chiller, 3 Phase to circulate refrigerant R-22A (which is built-in upon purchase) through a jacket lining the main hydrogen storage unit and the pipes that send hydrogen to the dispensing units. Built for outdoor industrial processing needs, this chiller contains a stainless steel pump and insulated poly tank built into a single cabinet. It is designed to properly cycle such that the compressor runs only when necessary in an effort to conserve energy. Installation will only require water in, water out, and a single point connection to electricity (Gwkent.com).
The refrigerant associated with this HVAC unit removes 120,000 BTU/hr from its target, in this case the pressurized hydrogen (Gwkent.com). Based on our heat duty calculations, this will require 0.4 m3 (or 400 kg) of R-22A. And, considering a typical HVAC efficiency 0.83 kW/ton (where a ton represents 12,000 BTU/hr), this system will require 8.3 kW to run continuously (Wastereductionpartners.org). Accordingly, it will have the capacity to keep the compressed hydrogen at -70˚C.
Safety Equipment
The module incorporates the Sinorix™ Waterless Fire Extinguishing system from Siemens (Siemens.com, a) that will be installed both in the canopy roofing the dispensing station as well as in the box that houses the main storage tank. This system has proven its ability to suppress a fire before it becomes large enough to warrant the activation of a traditional sprinkler system. Additionally, the Cerberus PRO fire alarm system from Siemens will be installed to serve both as an acoustical and visual warning (Siemens.com, b). It is estimated that these two units will cost approximately $4,100 (Siemens.com, a; Siemens.com, b; Hoodmart.com). If the electrical wiring system begins to contain too much current flowing through it (thus showing warning signs of shorting out), a Siemens Q330H circuit breaker will be connected to the system. It operates at 240 V and up to 30 A, and will cost approximately $100 (Circuitbreakerguys.com). In addition, lighting will be provided for hydrogen delivery at night as well as locks to access storage equipment.
Economic Analysis
Results
The primary financial measures of the success and feasibility of this project are whether the project will meet the 10-year payback period and the $2MM capital cost limit imposed by the contest. This proposal meets both of these criteria, costing only $189,000 in initial capital and paying back this initial investment in 8.0 years.
Discussion
The initial capital investment is limited to equipment and installation costs. The payback period is obtained by dividing this investment by the Net Income (NI), which is the sales revenue, less all costs. Direct costs of this proposal include to the cost of purchasing hydrogen and electricity usage of our equipment, while indirect costs include fixed yearly expenses such the rent on the station’s land and maintenance expenses. Sales revenue is estimated to yield $324,000, while annual costs amount to $296,355 per year. Thus, the yearly income of a single station is $27,645 (an ROI of 12.5%), and the payback period is 8.0 years.
Assumptions
For simplicity, this analysis ignores all taxes, restructuring costs, and other accounting complications, and thus Net Income is equal to Operating Income. This analysis also assumes the station sells 100 kilograms per day and operates 360 days per year. Because the gross profit is relatively low in magnitude, it is assumed that a corporation entering the hydrogen fuel market will construct this station, and only in areas where the consumer market is saturated.
Safety
Safety is an important aspect of our hydrogen dispensing station and care is taken to ensure the safety of our consumers and prolonged operation of our equipment. We will consider relevant codes and standards, identify potential risks with Failure Mode Analysis (FMEA), and derive appropriate safety procedure to mitigate risks.
Safety Codes and Standards
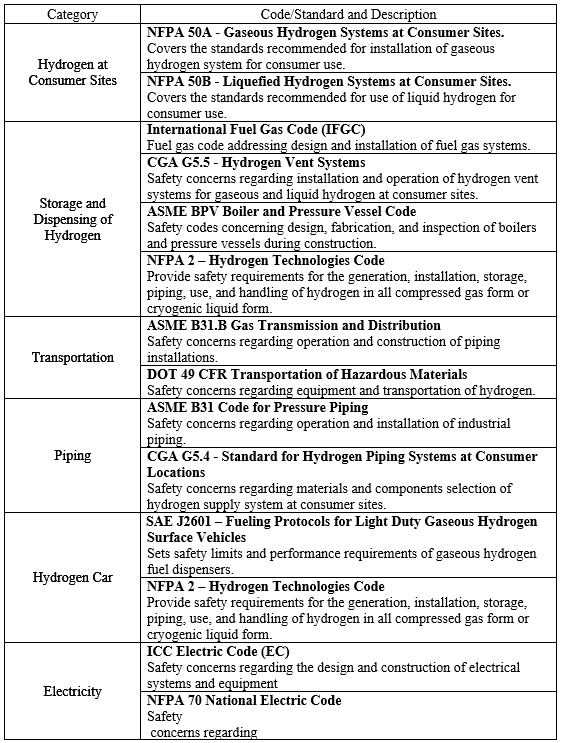
Safe operation and accident prevention are one of the primary objectives for design of hydrogen dispenser. In order to facilitate this objective, the safety codes and standards listed in the table below will be used to minimize injury to consumers, equipment, and infrastructure. The following safety considerations listed in this section will be our methods of adhering to the codes and standards to ensure the safety of our customers and equipment. A more exhaustive list of codes and standards can be found in reference (US Department of Energy, 2004).
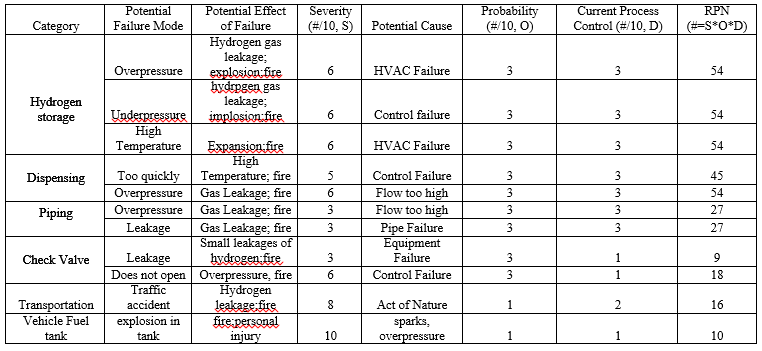
Failure Modes and Effects Analysis (FMEA)
The Failure Mode and Effects Analysis (FMEA) is an important analysis tool to help identify areas of risk in a process. The template for the following FMEA comes from ASQ (Asq.org) with the information from the actual analysis derived from other FMEA analysis on fuel dispensing station (US Department of Energy, 2004; Hydrogencontest.org). From our FMEA, we have identify considerable high risk areas associated with the storage equipment and dispensing station.
Safety Strategies for Risk Mitigation
From our FMEA, we have identify considerable high risk areas associated with the storage equipment and dispensing station. Using the appropriate codes and standards, we have derived the following safety strategies. To prevent single-point failure, we aim to provide redundancy in our safety equipment to further minimize risk.
Hydrogen quality either purchased or produced will follow CGA standards for commercial specification to fuel grade hydrogen and prevent fuel cell damage. Storage and piping will adhere to ASME standards for pressure vessels and piping. Pressure and temperature will be monitored using sensors installed on all storage and piping equipment. Storage tanks will be designed to prevent pressure from rising 10% above maximum working pressure. In the event of overpressurization, a pressure relief device consisting of a frangible disk to a relief valve. Adhering to CGA G5.4, NFPA 50A chapter 2, NFPA 50 B chapter 2, pressure relief valves will be installed for all systems with the potential of overpressurization. Vents will point towards where hydrogen cannot be trapped as well as withstand ice, wind, and seismic variations. Storage devices will separated 20 ft away from dispensing station and separated with a 5 ft high fire barrier. Storage vessels will be open-top to minimize accumulation of hydrogen. The location of the dispensing station will not be beneath power lines and far away from other flammable and/or sparking material. Flammable gas detectors will be located near pipe/storage junctions to set off alarms at 1% hydrogen using visual and audio recognizable alarms to warn consumers of possible hydrogen leaks.
Safety code relating to distribution will hold similarities with more common and established gasoline dispensing stations. Breakaway mechanism will be incorporated into the hose to prevent unrestricted hydrogen leakage in the event the dispensing equipment is still attached to a vehicle when the vehicle leaves the station. A maximum of two cars will be allowed into the dispensing station. Hydrogen sensors will be installed around the station to alert possible possible hydrogen leakage. Sensors will go off if it reaches to above 4% of lower flammable limit of air. In addition, "NO SMOKING, NO OPEN FLAMES" will be clearly displayed to minimize potential fire hazards due to consumers. Compressed hydrogen storage will be locked to minimize tampering of non-consumer equipment. The user interface equipment will also instruct consumers to safe areas away from the dispensing station during emergencies.
In addition, all communication between site and operation headquarters will be encrypted with RSA cryptosystem to prevent cyberattacks. Transportation of hydrogen will follow ASME and DOT codes. In case of emergencies, first responders and NVFEL personnel will be contacted for containment and removal.
Siting, Permitting, and Maintenance Codes
Siting
General Siting
During the initial search for the fuel station site, a review of the pertinent literature was performed. There are strict regulations concerning the local zoning requirements, transport of hydrogen to the site, and transfer to the station from the fuel truck (HyApproval WP2, 2008). The route to the site must not impede hydrogen shipment, so locations near major highways and larger thoroughfares were preferentially considered. Additionally, there needs to be room at the site for the truck to position and unload hydrogen. The only production equipment with major required safety distances is the high pressure gas storage system. These tanks need to be at least 25 feet from neighboring buildings that do not have sprinkler systems1.
Detroit, Michigan
Following the rules outlined in the general section, the site chosen was 2580 S Schaefer Highway in Detroit, Michigan. This lot is fairly large, 280x100 ft., and has the hookups available to support a car wash and/or oil change center (therefore, water and electricity systems can be used here). The initial capital investment here is substantial at ~$80,000, but insignificant compared to the total cost incurred from the compressors and the high pressure storage tanks. An idea of the surrounding area can be seen in the snapshot below (Trulia.com).
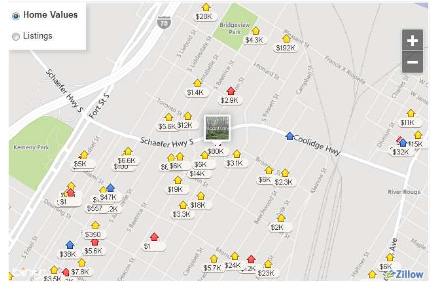 Figure 3. Proposed Site for Hydrogen Fueling Station
Figure 3. Proposed Site for Hydrogen Fueling Station
It can be inferred from Figure 3 that the fueling station will be highly accessible to both customers and suppliers alike. Being only 1-2 miles off a major expressway, Interstate 75, consumers will be able to find the location easily. Because this lot is on the corner of Schaefer Hwy, there should be room to accommodate fueling operations regardless traffic and congestion.
Permitting
Commercial construction requires adherence to the building permit checklist produced by the Buildings & Safety Engineering Department (BSED) of Detroit, MI. First, the design must meet zoning (Detroitmi.gov) and construction codes (Building, Residential, Mechanical, Electrical, Plumbing, and Uniform Energy codes). A Building Permit Application, which includes the project location and description, property legal description and owner information, our personal information, and notarized signatures of both our organization and the owner, must be prepared and submitted. The major sections of the construction document are the site plan, architectural plan, plumbing and mechanical plan, electrical plan, and structural and detail drawings.
Site Plan
- Address of our hydrogen fueling station
- Size and shape of the lot with property lines identified and all buildings and structures shown
- The distances between these components
- The infrastructure of our utilities systems
Architectural Plan
- Building information block noting the area of each building and their capacities, specifications on sprinklers, fire alarms, exits
- Detailed floor layout with equipment, dimensions of the rooms, all doors and windows, and heights of all the walls
- Location of restrooms
Plumbing and Mechanical Plan
- Complete floor plan of the mechanical layout, including ductwork, ventilation system (and associated mechanical calculations), etc.
- Floor plan showing restrooms, sinks, water closets with plumbing isometric drawings
- Roof and site drainage calculations
- Gas pipeline isometric drawing
Electrical Plan
- Locations of the Service Connection and each sub panel
- Lighting floor plan, power floor plan showing switches, outlets, etc.
- Drawing of the complete electrical system, including the service voltage, ampacity, phases, and overcurrent devices, maximum available fault current, sizes and types of wire, with grounding detail
- Exterior lighting plan
- Size of main breaker
- Location of any hazardous areas
Structural and Detail Drawing
- Foundation, floor framing, roof framing plans
- Cross-sectional views
- Connection details, calculations, soils report
- Wall details
- Material list for finishes
- Door and window schedules
- Hardware schedule
Operations and Maintenance
Operations
At this stage of the design process, specifics about the operation of individual process equipment are unnecessary. However, the general plan for training the remote operator is critical. Technical training concerning the potential hazards of hydrogen, safety regulations, and emergency procedures is necessary (HyApproval WP2, 2008). Once specific vendors are selected, detailed operating instructions will be provided based on the operating manual and industry best practices.
Maintenance
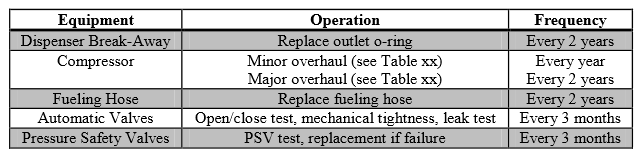
Due to the lack of on-site personnel, active preventative maintenance will be crucial to ensure safe operations. Since there are strict flammability concerns, the facility grounds must be free of debris, weeds, and other rubbish (HyApproval WP2, 2008). If there is grass near the site, it must be regularly cut to prevent the accumulation of rotting grass, which is capable of self-ignition. In terms of process safety, the flexible fueling hoses appear to be the most probable hazard1. Therefore, preventative maintenance in this area will require high scrutiny. Based on initial studies done prior to establishing hydrogen fueling stations in Europe, a sample maintenance plan can be seen in Table 1 (HyApproval WP2, 2008).
Table 1: Proposed Maintenance Plan for High Pressure Fueling Station
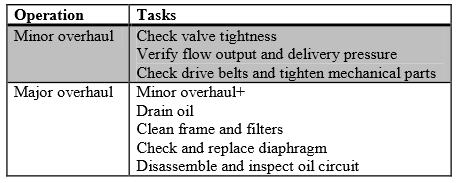
Because the high pressure compressors are the main wear pieces in the fueling process, great care needs to be taken. The contents of the stated overhaul modes are shown in Table 2 (HyApproval WP2, 2008).
Table 2: Inspection Plans for Dispensing Compressors
Environmental Analysis
One of the principal driving forces for using hydrogen fuel cells is the fact that byproducts of the energy-producing step are just water, eliminating the emission of carbon-based greenhouse gases. However, it is important to perform an environmental analysis of the process to ensure that the entire process of using hydrogen gas for fuel is superior to other technologies. Resource consumption, emissions, and noise were analyzed to determine the environmental impact of this process.
Resource Analysis
In order to transform the feedstock hydrogen into fuel condition hydrogen, this process consolidates all of its resource needs into electricity drawn from the local electrical grid. In this section, the thermodynamic efficiency for each piece of process equipment, as well as the overall process efficiency are calculated.
To determine the overall efficiency of this process, an energy balance was performed on each module. The general form of an energy balance is shown below:
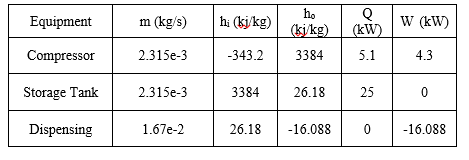
where i refers to the inlet, o refers to the outlet, indicates a mass flow rate and refers to the enthalpy per unit mass, Q is the rate of heat transfer, and W is the work performed on the system. Because the equipment operates in steady state, . Table 3 contains the values that were used in performing this calculation across each piece of process equipment, with enthalpy, Q and W values determine using the Peng-Robinson equation of state implemented in AspenTech HYSYS Process Simulation software, with inlet and storage conditions as stated above. The outlet conditions were assumed to be -40 degrees C, 200bar, as this is the largest temperature gradient that will be experienced during dispensing, and therefore maximizes inefficiencies.
Table 3: Energy Balance Values
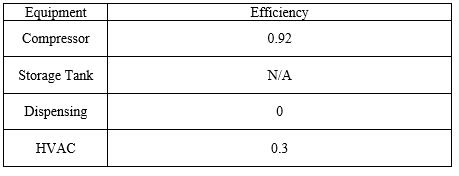
All values of heat transfer rates are ultimately turned into work done by the HVAC unit, which has an efficiency factor given by the manufacturer.
The efficiency of a given piece of equipment is given by the following expression:
where D is the duty of the process equipment, either as work or heat transfer, required to produce one kg of fuel grade H2 gas. The efficiency of each piece of equipment can be found in Table 4.
Table 4: Energy Balance Values
The total process efficiency factor is the total energy used to produce a single kg of H2 fuel divided by the minimum energy required by thermodynamics, or the following:
The value of η was determined to be 1.5.
Finally the total energy requirement to produce one kilogram of hydrogen fuel at dispensing conditions was found to be approximately 19,500kj. The energy density of hydrogen gas was found to be 120,000 kj/kg (Towler and Sinnott, 2013a). Based on this value, the process efficiency was calculated as the usable energy per kg of hydrogen divided by that value plus the processing energy as shown below:
ε was found to be 0.86.
Emission Analysis
Following the design decision to deliver hydrogen to the fueling station from outside sources, emissions analysis was conducted using the centralized hydrogen production document from the US DoE hydrogen analysis1. The state of the art technology for large companies in this field is to produce hydrogen using coal gasification followed by catalytic reforming. A second water gas shift reactor is used to convert remaining water to hydrogen (and carbon monoxide to carbon dioxide) 1. A significant amount of the carbon dioxide produced is sequestrated before the hydrogen stream is compressed for delivery.
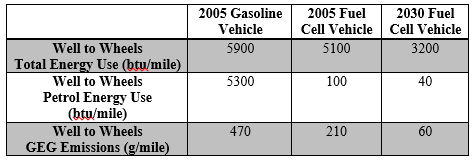
The DoE analysis was performed to include estimates for downstream energy costs at the hydrogen fueling station. Their final calculated values can be seen in Table 5, and significant discrepancies between their analysis and our design plan are detailed below.
Table 5 (US Department of Energy). Well to Wheels Energy and Greenhouse Gas (GEG) Data
It should be noted that the basis used in these calculations was for a 350 bar fueling tank. Therefore, the total energy use and GEG emissions are slight underestimations, because additional work is needed to compress the gas to 1000 bar during storage and 700 bar during fueling. Here, 85% of carbon dioxide produced during hydrogen production was assumed to be sequestered. This appears to be overly optimistic for most large scale firms; so again, the GEG emissions shown here are likely a significant underestimate.
Noise Analysis
The major sources of noise produced by this process are the compressor, the HVAC unit, and the expansion valves in the dispenser unit.
Of these three pieces of equipment, the compressor is the loudest, with a high-end estimate of 95dB at a distance of 1 meter from the equipment. A high-end estimate for the sound output of an industrial strength cooling unit is 70dB (Airconco.com). Adding these two sound signals together results in a total output of 95.04dB. This justifies neglecting all sound output relative to the compressor.
Because sound energy dissipates proportional to the distance from the source cubed, and other safety requirements require a minimum of 8 meters separation between users and the sound source, no users are in immediate danger. A rule of thumb for calculating sound reduction is that sound reduces by 6dB per doubling of distance between the source and observer. Therefore, at 8 meters, the sound of the compressor would be reduced to 77dB (Sengpielaudio.com), which is well below safety limits for long-term exposure (Airconco.com).
To further reduce noise for the benefit of neighbors to the process, a low cost sound containment wall that will reduce the noise output by 20dB across the 2” barrier can be purchased for under $1000 (Zoro.com).
Therefore, with appropriate sound containment equipment installed, noise pollution is not anticipated to be a concern.
Consumer Interface and Education
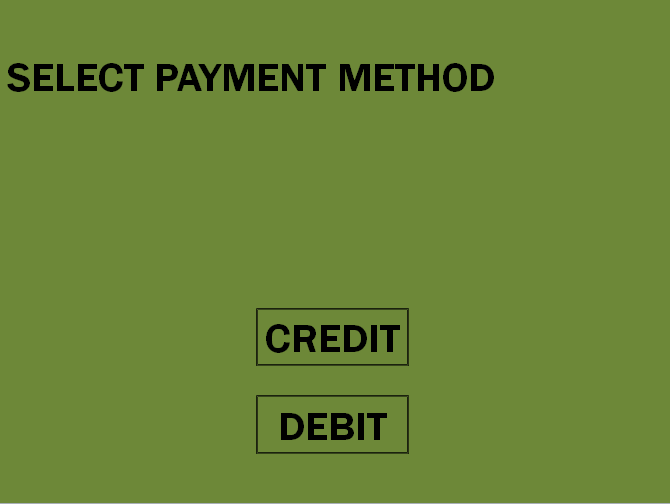
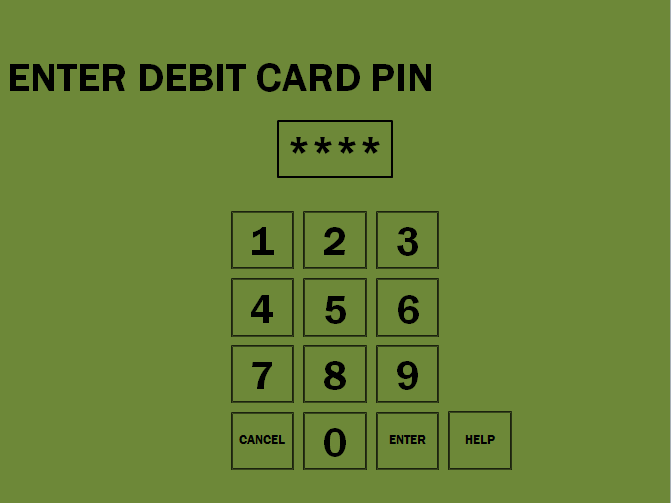
The hydrogen fuel dispenser is not a novel apparatus; in fact many companies have developed their own hydrogen dispensers to be used specifically in hydrogen fueling stations such as the one designed and proposed in this report (Figure 7-1). These dispensers have been designed to mimic the conventional consumer experience at the “gas station” as closely as possible in order to maximize consumer comfort in incorporating the new technology and minimize a possible “learning curve” (Airproducts.com).
Fuel Dispenser Visual Interface
In view of these efforts, however, certain aspects of the consumer experience at the hydrogen fueling pump will be intrinsically different as a result of the nature of hydrogen fuel. Firstly, it is the industry standard that hydrogen fuel loaded be reported on fueling apparatuses in kilograms and its price in dollars per kilogram; having the units be the conventional gallons and dollars per gallon as seen with gas stations would not only burden suppliers but also consumers. Furthermore it is of importance to note that the hydrogen fueling pump is a closed process – specifically, the dispensing nozzle needs to be attached to the consumer vehicle in a matter which is not open to the atmosphere and meets a long list of standardized requirements, unlike its “gas station” counterpart (Sae.org).
 Figure 4: Air Products hydrogen fueling pump.
Figure 4: Air Products hydrogen fueling pump.
Due to the nature of hydrogen fuel, fuel grade is no longer of relevance to the consumer experience. This is the case because hydrogen gas is the energy carrier to be utilized by all vehicles and no additives are included in the gas supplied to consumer vehicles. Additionally, due to the mobile and unmanned nature of the fueling station proposed in this report, the option of a car wash, which would also impact the per-kilogram price of hydrogen fuel, is also not planned to be available to the consumer. All of the mentioned changes to the conventional fueling station experience will be reflected in the customer visual interface by way of additional instructions, or lack thereof, for the case of nozzle fastening and car wash availability respectively (see Appendix B).
Consumer Education Strategy
To further enhance existing consumer education efforts in preparation for the eventual widespread incorporation of hydrogen fuel for consumer transportation needs, a tri-fold brochure design was created. The brochure incorporates information about hydrogen fuel and its potential for the future and a special effort was made to keep the publication-ready brochure as purely educational. This means that no propagandizing about hydrogen fuel was allowed – all information was provided in a balanced and factually sound manner, with reputable sources being the only sources acceptable for the ask and cited properly on the brochure itself (see Appendix C).
The tri-fold brochure is to be strategically located in places where consumers will have sufficient time to invest to reading the brochure and learning from it. For drivers of all ages, the Department of Motor Vehicles offices would be ideal locations to stock with hydrogen fuel informational brochures such as the one designed. For young and soon-to-be drivers, the brochure would be made available at the driving schools consumers of the age group are required to attend and also high schools which are capable of instructing their own driving courses.
Appendix A: Calculation Calculations
Appendix B: Pump and Interface Touch Screen Display Logic
Appendix C: Hydrogen Fuel Brochure
References
Airconco.com. Decibel Scale - Amazing Decibel Infographic [Internet]. London: Airconco (UK) Ltd T/A The Air Conditioning Company [cited 2014 Mar 6]. Available from: http://www.airconco.com/decibel_scale/.
Airproducts.com. News Release: Air Products Introduces Advanced Retail Hydrogen Fuel Dispenser [Internet]. Allentown: Air Products and Chemicals, Inc.; c1996-2015 [cited 2014 Mar 5]. Available from: http://www.airproducts.com/company/news-center/2013/06/0611-air-products-introduces-advanced-retail-hydrogen-fuel-dispenser.aspx.
Alibaba.com [Internet]. Hangzhou: Alibaba.com; c1999-2015 [cited 2014 Mar 12]. Available from: http://www.alibaba.com/showroom/hydrogen-storage-tank.html.
Asq.org. Failure Mode Effects Analysis (FMEA) [Internet]. Milwaukee: American Society for Quality [cited 2014 Mar 7]. Available from: http://asq.org/learn-about-quality/process-analysis-tools/overview/fmea.html.
Circuitbreakerguys.com. Q330H Circuit Breaker by Siemens [Internet]. Burlingame: Circuit Breaker Guys LLC.; c2014 [cited 2014 Mar 7]. Available from: http://www.circuitbreakerguys.com/q330h/?gclid=CM_P2pbJgb0CFbFaMgod_hUAIw.
Couper JR, Penney WR, Fair JR. Rules of Thumb Summary. In: Chemical Process Equipment: Selection and Design. 3rd ed. Boston: Elsevier; 2012.
Cumalioglu I, Ma Y, Ertas A, Maxwell T. High Pressure Hydrogen Storage Tank: A Parametric Study. J Pressure Vessel Technol. 2006 Apr;129(1):216-222.
Detroitmi.gov. Building Codes/Construction Information: Commercial Building Permit/Submittal Checklist [Internet]. Detroit: City of Detroit; c2001-15 [cited 2014 Mar 6]. Available from: http://www.detroitmi.gov/DepartmentsandAgencies/BuildingsSafetyEngineeringEnvironmental/Divisions/LicensesPermits/Permits.aspx.
Gwkent.com. 10 ton Glycol Chiller 3-Phase [Internet]. Ypsilanti: GW Kent; c2010-15 [cited 2014 Mar 12]. Available from: http://www.gwkent.com/10-ton-glycol-chiller-3-phase.html.
Hoodmart.com. Restaurant Fire Suppression Systems: Wet Chemical & Pre-Piped [Internet]. Grafton: HoodMart Inc. [cited 2014 Mar 7]. Available from: http://www.hoodmart.com/restaurant-fire-suppression-system-pfs19.aspx?utm_medium=shoppingengine&utm_source=googlebase&gclid=CIrkm5nHgb0CFeZDMgodq38ASA#.UxpTaF5kLGo.
HyApproval WP2. Handbook for Hydrogen Refueling Station Approval [Internet]. [updated 2008 June 4; cited 2014 Mar 7]. Available from: http://www.hyapproval.org/Publications/The_Handbook/HyApproval_Final_Handbook.pdf.
Hydrogencontest.org. 2011 Contest Residential Fueling with Hydrogen [Internet]. Washington, D.C.: Hydrogen Student Design Contest [cited 2014 Mar 7]. Available from: http://www.hydrogencontest.org/2011.asp.
Hydropac.com. High Pressure Hydrogen Compressors [Internet]. Fairview: Hydro-Pac Inc. [cited 2015 Mar 1]. Available from: http://www.hydropac.com/HTML/hydrogen-compressor.html.
Keytometals.com. Steels for Cryogenic and Low-Temperature Service [Internet]. Zürich: Key to Metals AG; c2015 [cited 2015 Mar 1]. Available from: http://www.keytometals.com/page.aspx?ID=CheckArticle&site=kts&NM=61.
Sae.org. Compressed Hydrogen Surface Vehicle Refueling Connection Devices. [Internet]. Warrendale: SAE International; c2015 [cited 2014 Mar 5]. Available from: http://standards.sae.org/j2600_201211/.
Sakintuna B, Lamari-Darkrim F, Hirscher M. Metal hydride materials for solid hydrogen storage: A review. Int J Hydrogen Energy. 2007 June;32(9):1121-40.
Sandler SI. Chemical, Biological, and Engineering Thermodynamics. 4th ed. Hobeken: John Wiley and Sons; 2006.
Sengpielaudio.com. Damping of sound level (decibel dB) vs. distance [Internet]. London: Airconco (UK) Ltd T/A The Air Conditioning Company [cited 2014 Mar 6]. Available from: http://sengpielaudio.com/calculator-distance.htm.
Sculley J, Yuan D, Zhou H. The current status of hydrogen storage in metal-organic-frameworks-updated. Energy and Environ Sci. 2011 June;4:2721-35.
Siemens.com. Fire Suppression Systems [Internet]. Munich: Siemens AG; c1996-2015a [cited 2014 Mar 7]. Available from: http://w3.usa.siemens.com/buildingtechnologies/us/en/fire-products-and-systems/fire-suppression-system/Pages/fire-suppression-system.aspx.
Siemens.com. Cerberus PRO – An Intelligent Fire Protection System [Internet]. Munich: Siemens AG; c1996-2015b [cited 2014 Mar 7]. Available from: http://w3.usa.siemens.com/buildingtechnologies/us/en/fire-products-and-systems/fire-alarm-systems/cerberus-pro/Pages/cerberus-pro.aspx.
The American Society of Mechanical Engineers. Section VIII. In: Boiler and Pressure Vessel Code. New York: ASME; 2004.
Towler G, Sinnott R. Safety and Loss Prevention. In: Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013a.
Towler G, Sinnott R. Design of Pressure Vessels. In: Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013b.
Trulia.com. 2580 S Schaefer Hwy [Internet]. San Francisco: Trulia, Inc.; c2015 [cited 2014 Mar 7]. Available from: http://www.trulia.com/property/1033919029-2580-S-Schaefer-Hwy-Detroit-MI-48217.
Wastereductionpartners.org. Chillers: Energy Saving Fact Sheet [Internet]. North Carolina Energy Office [cited 2014 Mar 7]. Available from: http://wastereductionpartners.org/phocadownload/userupload/Resources/Energy_Saving_Fact_Sheet_Chillers.pdf.
Wikipedia.org. Hydrogen Embrittlement [Internet]. Wikimedia Foundation [cited 2015 Mar 1]. Available from: http://en.wikipedia.org/wiki/Hydrogen_embrittlement.
US Department of Energy. Module 2 Permitting Hydrogen Motor Fuel Dispensing Facilities. Version 1.0. Pacific Northwest National Laboratory. Report No.: PNNL-14518. 2004 Dec.
US Department of Energy. Centralized Hydrogen Production from Coal Gasification with Sequestration [Internet]. [cited 2014 Mar 6]. Available from: www.hydrogen.energy.gov/docs/cs_central_coal_gasification.doc.
Zoro.com. Noise Control Products [Internet]. Buffalo Grove: Zoro Tools, Inc.; c2011-15 [cited 2014 Mar 5]. Available from: http://www.zorotools.com/g/00060581/k-G2499777?utm_source=google_shopping&utm_medium=cpc&utm_campaign=Google_Shopping_Feed&kw={keyword}&gclid=CPmd_MG9_LwCFaw-MgodQzYAHQ.