Ethanol to Ethylene (B1): Difference between revisions
| (51 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:BFD_B1.png|600px|thumbnail|alt=Alt text|Conversion of Ethanol to Ethylene]] | [[File:BFD_B1.png|600px|thumbnail|alt=Alt text|Conversion of Ethanol to Ethylene]] | ||
Team B1 Final Report | |||
Authors: Harry Poppick, Emm Fulk and Scott Smith | |||
Instructors: Fengqi You, David Wegerer | |||
March 13, 2015 | |||
=Executive Summary= | =Executive Summary= | ||
| Line 9: | Line 18: | ||
=Introduction= | =Introduction= | ||
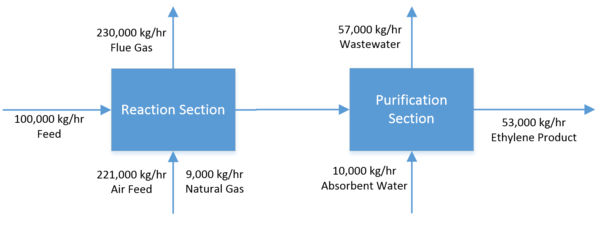
Ethylene is a critical chemical precursor to a number of different industrial products. It is often derived from petroleum sources and polymerized to polyethylene, one of the most ubiquitous plastics today. (American Chemistry Council, 2013) Recently there has been a significant effort to develop production pathways utilizing biologically-derived feedstocks, such as corn or cellulosic ethanol, rather than traditional fossil-fuel sources. Ethylene can be produced from bioethanol via catalytic dehydration over an aluminum oxide catalyst. The proposed process converts 100,000 kg/hr of 95 wt% ethanol, 5% water feed to 53,000 kg/hr of 99.7% chemical-grade ethylene when operating at full capacity. This process was found to be economically viable with a 10-year net present value (NPV) of $131 million (2014 USD) and a payback period of approximately 2.7 years. This memo will detail the proposed process, as well as outline potential alternatives and economic viability of the project. | |||
=Technical Approach= | =Technical Approach= | ||
Many bioethanol plants are located in the Midwest where large amounts of feedstock crops, such as corn, are grown. As such, this plant will be located in Story county, Iowa, near a large-scale bioethanol production plant. (DuPont Nevada Site, 2012) It is assumed that the supply of bioethanol is consistent and readily available. It is also assumed that other reagents - namely, the aluminum oxide catalyst, heater fuel, utility water and freon refrigerant - are readily available, and that wastewater treatment facilities are located near enough to facilitate the off-site treatment of wastewater. Plant capacity was based on current mid-sized industrial ethylene plants. (Designing Ethylene Plants, 2014) | |||
HYSYS simulations were performed with the NRTL fluid package and Peng-Robinson vapor model. The NRTL fluid package is an activity coefficient model that is especially useful to model the separations of alcohols in aqueous solutions. (Suppes, 2015) In addition, NRTL uses models of binary pairs of components, much like the Wilson equation, to predict chemical interactions at the molecular level. The NRTL package can thus be easily extended to ternary and higher order chemical systems, making it ideal for this type of simulation. The Peng-Robinson vapor model was selected as it is well-suited to account for the non-idealities of the vapor phase reactions and separations integral to this process. (ASPENtech, 2014) | |||
It was assumed that the feed enters the process at ambient temperature and pressure (25 °C, 1 atm) and at a consistent rate and composition with negligible quantities of impurities. The ethanol feed was assumed to be 95% ethanol and 5% water by mass, which is the commercial standard for industrial ethanol. (190 Proof Ethanol, 2012) Storage vessels for the feed ethanol, waste streams and products are assumed to be located nearby to the process. An initial feed pump compensates for pressure drops through the process and reduces the duty on downstream compressors. (Hadawey, 2015) | |||
Natural gas, assumed to consist of 83% methane, 16% ethane and 1% nitrogen by mass, was supplied to two fired heaters at a flow rate estimated by HYSYS. (Methane, 2015) Air fed to the process to enable combustion was assumed to be 23% oxygen and 77% nitrogen by mass. (Composition of Air, 2015) Air streams were fed at 50% molar excess in order to ensure complete combustion and prevent the formation of carbon monoxide in the heater exhaust. Total flue gases are assumed to be 230,000 kg/hr, or 2.2 million tons annually, and consist of 73.8% nitrogen gas, 10.5% carbon dioxide, 8.2% water vapor, and 7.5% oxygen by mass. The only compound of concern in these emissions is the carbon dioxide, a known greenhouse gas. At the time of this report, the quantities of carbon dioxide emitted by this plant annually are within acceptable EPA specifications. (Summary of the Clean Air Act, 2015) | |||
Due to the difficulty of simulation equilibrium and conversion reactions in a single vessel in HYSYS, these two reaction types were simulated in separate equilibrium reactor and conversion reactor units in series. In addition, while HYSYS indicated that reactor units would output separate vapor and liquid streams, it was assumed that in practice this would take the form of a single, two phase stream between each unit. To account for this, mixers were installed downstream of each reactor to combine the liquid and vapor phases of the reactor effluent. The three consequent reactions considered are as follows: | |||
[[File:Equation.PNG|300px|center]] | |||
The optimal reaction temperature was determined to be 400 °C, which promotes the equilibrium reaction of ethanol to ethylene while minimizing byproducts. (Zhang and Yu, 2013) While other byproducts may be produced in this process, these reactions are typically taken to comprise of the bulk of the dehydration reaction. Heat losses over these reactors were assumed to be negligible. (Zhang and Yu, 2013)All major process equipment is assumed to be constructed of high strength carbon steel. (AspenTech, 2012) | |||
The “Sizing Utility” within the Icarus Economic Evaluation module was used to estimate sizes of major equipment based on the process simulation. These values can serve as a benchmark for an initial plant design. Icarus KbaseTM implements a wide variety of mechanical design standards, including ASME and BS5500, in order to calculate accurate equipment sizing. The Icarus sizing module combines equipment prices for standard chemical processing equipment as well as correlations and sizing factors. The advantage of using a software package such as HYSYS to price out individual equipment is that it utilizes up-to-date prices from vendors and distributors when estimating costs. A more in-depth overview of the correlations included in this software can be found in Towler and Sinnott (2009). | |||
=Process Flowsheet= | =Process Flowsheet= | ||
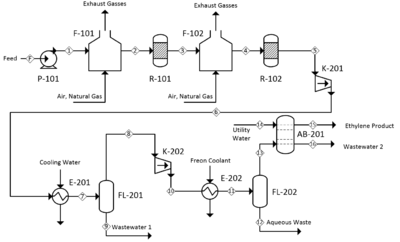
[[File:Squarepfd_B1.PNG|thumb|400px]] | [[File:Squarepfd_B1.PNG|thumb|400px|alt=Alt text|Fig 1: PFD of the Proposed Process]] | ||
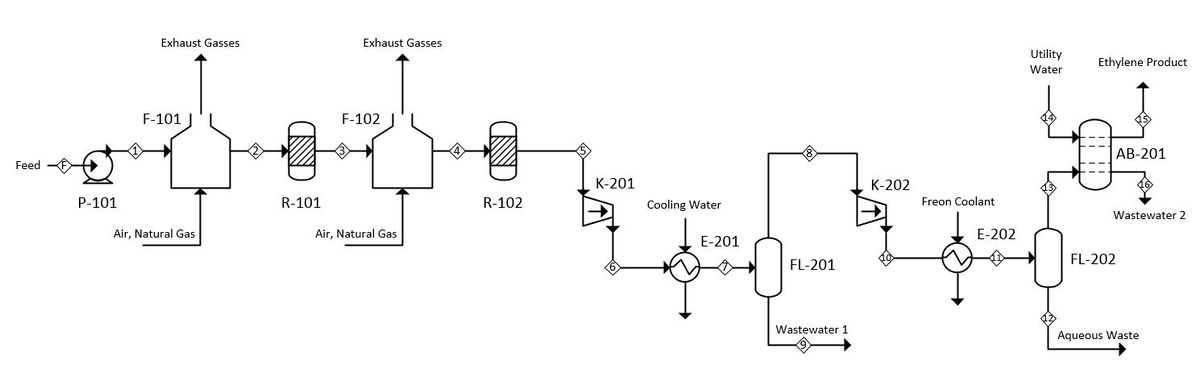
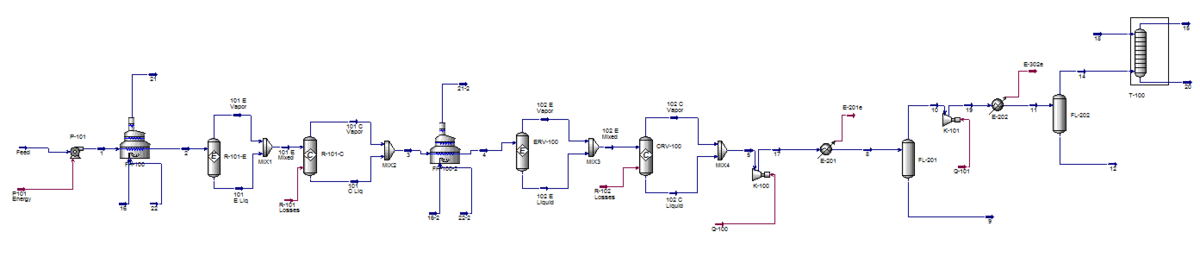
The following process is based primarily on a 1983 US patent titled “Process for dehydration of a low molecular weight alcohol.” (Barrocas and Baratelli, 1983) The process flow diagram can be seen in Figure 1. A larger version of the PFD is included in Appendix I and the HYSYS simulation setup in Appendix II. | |||
The feed enters the process at a rate of 100,000 kg/hr, a temperature and pressure of a 25 °C and 1 atm and at a composition of 95 wt% ethanol and 5 wt% water.It is initially pressurized to 4335 kPa by centrifugal pump P-101 and subsequently heated to 400 °C by fired heater F-101. The heated and pressurized feed enters fixed bed reactor R-101 and contacts with the aluminum oxide catalyst. At this point it dehydrates to form the product, ethylene, as well as the byproducts water, hydrogen, diethyl ether and acetaldehyde. Fired heater F-102 and reactor R-102 increase the overall conversion of the process. Both fired heaters F-101 and F-102 are fueled with natural gas and fed with excess air. The composition of the reactor train effluent is 54.5 wt% ethylene, 37.1 wt% water, 8.1 wt% unreacted ethanol and trace amounts of diethyl ether, acetaldehyde, and hydrogen contaminants. | |||
The pressure of the reactor train effluent is increased to 4500 kPa by compressor K-102 and the temperature decreased to 100 °C by exchange with cold water in heat exchanger E-201. The stream then expands and flashes in flash vessel FL-201, which separates out 82.3% of the unreacted ethanol, 96.6% of the water and virtually all of the acetaldehyde contaminant in the bottoms while sending the ethylene-rich vapor phase out of the top. The top product of FL-202 is compressed to 3500 kPA by K-202 and cooled to 0.0 °C by a freon refrigerant in E-202. At this point the stream is flashed for a second time in vessel FL-202 to remove additional impurities. The top outlet stream consists of 99.5 wt% ethylene, which is fed to the bottom of a 10-tray absorption column that removes a fraction of the residual diethyl ether as well as the remainder of the unreacted ethanol. A stream of absorbent water is fed to the top of the column at 10,000 kg/hr. The product exits the top of the column at a rate of 54,600 kg/hr and has a purity of 99.7% ethylene. Diethyl ether makes up 0.2% of the remaining product stream and is the primary contaminant. Trace amounts of residual water and hydrogen are also present. | |||
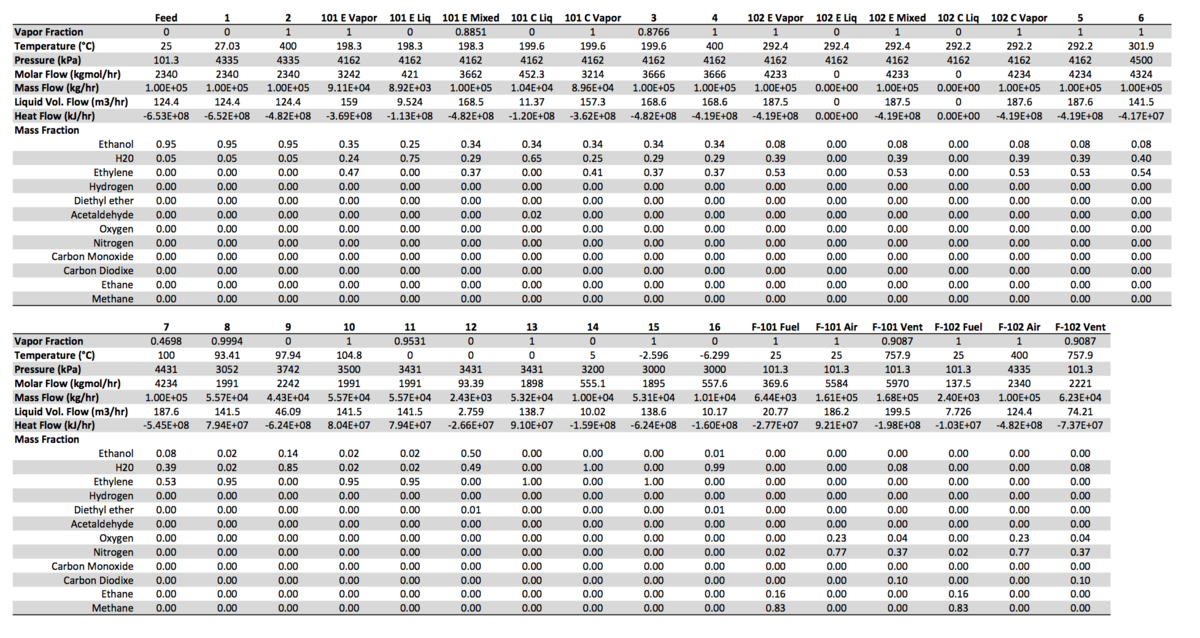
Full tables of material and energy streams can be found in Appendices III and IV respectively. | |||
==Process Alternatives== | |||
===Reactor Alternatives=== | |||
Two fixed bed reactors, alternating with fired heaters, are used in the dehydration reaction of ethanol to ethylene. However, a fluidized bed reactor can offer better mixing and temperature control than a fixed bed reactor and thus provide the highest possible conversion of ethanol with the greatest selectivity to ethylene. (Tsao and Zasloff, 1979) Fluidized bed reactors also provide a lower rate of catalyst coking and byproduct formation, minimizing separation and catalyst regeneration costs. (Morschbacker, 2009) The major downside of fluidized bed reactors is that few large-scale processes have been built since the technology was developed in the late 1970s. (Tsao and Zasloff, 1979; Morschbacker, 2009) Although implementation of fluidized bed reactors could potentially increase the purity of the ethylene product and/or decrease costs, a small scale pilot process would need to be built and tested before it would be possible to develop an industrial-scale facility. | |||
===Catalyst Alternatives=== | |||
Aluminum oxide, specifically gamma alumina, is the most industrially-common catalyst and has been historically used due to its low cost and high stability. (Tsao and Zasloff, 1979; Fan and Dai, n.d.) Recent improvements in selectivity and catalyst stability of activated alumina have been achieved by incorporating titanium oxide into the catalyst, although this increases reaction temperature by approximately 50 °C and decreases catalyst lifespan. (Chen and Li, et. al. 2007) There is also significant ongoing research into HZSM-5 zeolite and heteropolyacids, which are being explored as potential alternatives to alumina oxide. A nanoscale HZSM-5 zeolite catalyst has been shown to operate at the relatively low temperature of 200-300°C, with a 100% ethanol conversion and 99.7% selectivity to ethylene. Initial research has also indicated that it maintains greater than 98% selectivity for 630 operational hours. (Fan and Dai, n.d.) However, the current low cost and ready availability of unmodified alumina oxide makes it a better choice for a process of this scale. | |||
===Purification Alternatives=== | |||
As specified in this process, the reactor effluent is purified by two flash drums and absorption with water to produce chemical-grade ethylene. If chemical-grade ethylene is indeed the desired product, other purification options include caustic washing of the reactor effluent and water removal in a desiccant drying bed. (Morschbacker, 2009) While this purification train is relatively simple and does not require harsh chemical reagents, polymer grade ethylene is more commercially valuable and the additional cost of removing water and diethyl ether contaminants may be justified by increased revenue. Cryogenic distillation was initially simulated to produce polymer-grade ethylene, but utility requirements were found to render this separation economically unviable. (Barrocas and Baratelli, 1983) Further research and optimization may find this purification strategy to be a more valid process. Alternatively, the addition of a cryogenic turboexpander or the use of alternative adsorbents may increase product purity to polymer grade. (Jumonville, 2010) Initial HYSYS simulations indicate that these operations may be feasible with further refinement. | |||
===Wastewater Treatment=== | |||
Due to the large volume of wastewater produced, building an on-site wastewater treatment facility may prove to be more economical than paying for third-party wastewater treatment. Flash vessel FL-201 produces 44,600 L/hr of wastewater contaminated with 18.7 vol% ethanol and 0.6 vol% acetaldehyde, while FL-202 produces 2940 L/hr of an aqueous waste consisting of 56.8 vol% ethanol, 42.0 vol% water and 0.9 vol% diethyl ether. The absorption column produces approximately 10,200 liters/hr of wastewater contaminated with 1.2 vol% ethanol, 0.2% diethyl ether and 0.2% acetaldehyde. Utilizing distillation to separate these streams into clean water and volatile organic waste could ultimately reduce the quantity of waste that requires disposal and reduce the overall costs of the process. | |||
==Process Equipment== | |||
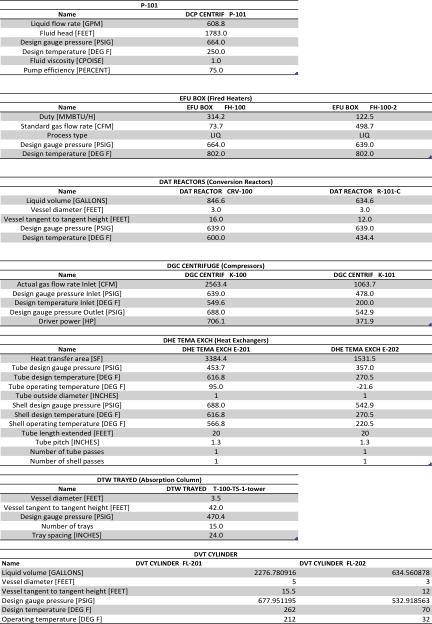
Relevant sizing information for each unit operation, estimated by the method described in the “Technical Approach,” can be found in Appendix V. Equipment and piping is assumed to be constructed of carbon steel. It should be noted that the two fired heaters are proportionately large enough to heat the entirety of the process stream and are thus larger than standard equipment. | |||
=Economic Analysis= | =Economic Analysis= | ||
[[File:sensitivityanalysis_B1.png|400px|thumb]] | [[File:NPV_B1.png|400px|thumb|Fig 2. Projected Cash Flow]] | ||
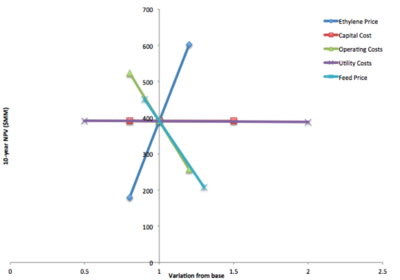
[[File:sensitivityanalysis_B1.png|400px|thumb| Fig 3. Results of the Sensitivity Analysis on 10 year NPV]] | |||
==Economics Overview== | |||
All final values are normalized to June 2014 USD by CEPCI scaling. It is assumed that all products produced are sold at current market prices. | |||
The Aspen Economic Evaluation module within HYSYS v8.0 was used to estimate capital and operating costs for a 10-year plant life span with a construction start date of April 2015. The price of the 95% ethanol feed stream was estimated at $1.44 per gallon, while the chemical grade ethylene produced is currently selling for approximately $1350 per metric ton. (Today in Energy, 2015; Weddle, 2014) Mapping and sizing calculations were performed by the economics module and a preliminary economic evaluation was completed. Equipment sizes, also estimated by HYSYS, were reviewed to ensure that height, diameter, and volume specifications were within realistic ranges. | |||
The process was ideally projected to operate 360 days per year with a start-up time of one year. In addition, the tax rate was approximated at 40%, an interest rate at 20%, and a salvage value of 20%. Escalation percentages were compounded yearly for the product, raw material, and operating costs. The total capital cost for this process is approximately $16.4 million, which includes all equipment and piping described in the process. The most expensive equipment used in this process are the two fired heaters F-101 and F-102, which have an exceptionally large heat exchange area to adequately heat the feed. Standard heaters may not suffice and additional costs may be incurred for the raw materials and manufacture of this equipment. Including a feed preheater or additional fired heaters may reduce this cost in subsequent design iterations. | |||
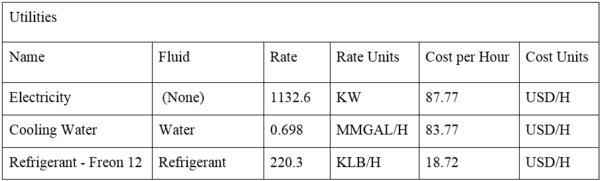
The total operating cost is approximately $465 million per year, about $427 million of which is the cost of the ethanol feed and natural gas for the two fired heaters. An additional $1.73 million per year is used for utilities including the cooling water and refrigerant. The labor and maintenance costs of this industrial process were also accounted for within the simulation’s economic analysis. The HYSYS simulation software, in conjunction with the Icarus economic analyzer, estimated there should be 5 operators per 8 hour shift with a cost per operator of $20 per hour. This results in a total operating labor cost of approximately $880,000 per year. In addition, the process will require one supervisor per shift with an hourly rate of $35 for total supervisor salaries of $307,000 per year. Finally, a fixed maintenance rate was estimated at $205,000 per 8000 hours of operation for a total cost of $225,000 per year. | |||
Total product sales are roughly $640 million per year at full operation. The estimated income for this process is thus approximately $175 million per year. After ten years, the plant is projected to have a net present value (NPV) of $391 million and an internal rate of return of approximately 79%. In addition the payout period for the initial investment is estimated to be about 2.7 years. Figure 2 shows the projected cash flow for the project. | |||
==Sensitivity Analysis== | |||
A sensitivity analysis was performed to investigate the effects of fluctuating capital costs, operating costs, raw materials, utility costs and ethylene sales price on the 10-year net present value (NPV) of the project. This analysis was performed by scaling these cost factors according to typical ranges given in Towler and Sinnott and calculating the adjusting the NPV accordingly. The results, illustrated in Figure 3, indicate that the project value is most sensitive to product sales, raw materials costs, and operating costs. Ethylene and ethanol prices are market-determined and cannot be controlled for in the process design, so further optimization should be focused on minimizing the operations cost of the process. (Today in Energy, 2015; Weddle, 2014) However, in feed and product prices do still have a profound effect on profitability and feasibility. A more rigorous market analysis will better inform the profitability outlook. | |||
=Conclusions/Recommendations= | |||
The proposed process produces approximately 460,000 metric tons of 99.7 wt% ethylene annually, with a 10-year net present value of $131 million. If current economic conditions hold, it is recommended that this project proceed to implementation following further design improvements and optimization. | |||
Future design iterations should focus on process optimization, particularly in minimizing operating costs as prescribed by the results of the sensitivity analysis. The development of a heat-exchanger network, potentially with Aspen Energy Analyzer, is one option for decreasing utility requirements and increasing the efficiency of the process overall. Optimization of the fired heaters could also result in decreased operational costs. Although overall profitability is less sensitive to capital costs, reviewing and adjusting equipment sizing would also improve the economic outlook. In particular, exploring alternative heating strategies at the beginning of the process could significantly decrease capital costs. Although there are not any particularly dangerous chemicals utilized in the process, an overall process review should also consider potential failure modes to minimize the safety risk to plant workers and nearby residents. | |||
In addition to economic and safety considerations, harmful emissions and waste streams should be minimized both to bring down the cost of treatment and to decrease environmental effects. The two fired heaters consume a large amount of natural gas per year, emitting about 200,000 tons of CO2 annually, and account for more than half of the capital cost of the process. Although no known toxic gases are produced, carbon capture or other emissions-reduction methods should be considered. Additionally, enough wastewater is produced by this process that building an onsite wastewater treatment center should be considered. This could not only decrease operating costs by eliminating the need for an external contract but also give the plant more control over the final purity of the treated wastewater. | |||
Finally, the current model assumes that all ethylene produced will be sold at a current prices and price or product sales will not be affected by economic variability. A more detailed study of the chemical-grade ethylene market, as well as a more thorough understanding of sales and distribution logistics, would increase the surety of a profitable process. | |||
In summary, the proposed process yields chemical grade ethylene via the catalytic dehydration of ethanol. A 100,000 kg/hr of 95 wt% ethanol, 5% water feed is converted to 53,000 kg/hr of 99.7% chemical-grade ethylene. After 10 years of operation, including a one-year startup time, the process has an NPV of $391 million with an IRR of 79% and a payback period of 2.7 years. | |||
Pending further optimization, this process was determined to be viable and it is recommended for the project to move forward. | |||
=References= | =References= | ||
AspenTech. Appendix A - Property Methods and Calculations. Simulation Basics. 2014;A-3 - A-26. | |||
AspenTech. Aspen HYSYS Help File v8.0. Aspen Technology, Inc. 2012. | |||
Barrocas H, Baratelli F, inventors; Petroleo Brasileiro S.A., assignee. Process for dehydration of a low molecular weight alcohol. US patent 4,396,789 A. 1983 Aug 2. | |||
[ | Britannica.com. Methane (Chemical Compound) [Internet]. Encyclopædia Britannica Online; c2015 [cited 13 Mar 2015]. Available from: http://www.britannica.com/EBchecked/topic/378264/methane. | ||
Chen G, Li S, Jiao F, Yuan Q. Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors. Catal Today. 2007;125:111–119. | |||
[ | Composition of Air [Internet]. Engineering Toolbox; n.d. [cited 13 Mar 2015]. Available from: http://www.engineeringtoolbox.com/air-composition-d_212.html. | ||
[ | Designing Ethylene Plants. Ethylene [Internet]. Technip; c2014 [cited 13 Mar 2015]. Available from: http://www.technip.com/en/our-business/onshore/ethylene. | ||
DuPont Nevada Site Cellulosic Ethanol Facility [Internet]. DuPont Chemical; c2012-2015 [cited 11 Mar 2015]. Available from: http://biofuels.dupont.com/cellulosic-ethanol/nevada-site-ce-facility/. | |||
Epa.gov. Summary of the Clean Air Act, 2015 [Internet]. United States Environmental Protection Agency; c2015 [cited 13 Mar. 2015]. Available from: http://www2.epa.gov/laws-regulations/summary-clean-air-act. | |||
Fan D, Dai DJ, Wu HS. Ethylene Formation by Catalytic Dehydration of Ethanol with Industrial Considerations. Materials. 2012 Dec;6:101-115. | |||
Jumonville, J. Tutorial on Cryogenic Turboexpanders. Proceedings of the Thirty-Ninth Turbomachinery Symposium; 2010 Oct 4-7; Houston, TX. Houston: Texas A&M University; 2010. | |||
Morschbacker, A. Bio-Ethanol Based Ethylene. Polymer Reviews. 2009; 49:79-84. | |||
Statista.com. Production of chemicals and plastics in the U.S. in 2013, by type (in 1,000 metric tons) [Internet]. American Chemistry Council; c2014 [cited 15 Jan 2015]. Available from: http://www.statista.com/statistics/299725/total-us-plastics-and-chemicals-shipments-by-type/. | |||
[ | Suppes GJ. Selecting Thermodynamic Models for Process Simulation of Organic VLE and LLE Systems [Internet]. The University of Missouri-Columbia, Dept of Chemical Engineering; c2002 [cited 13 Mar 2015]. Available from: http://web.missouri.edu/~suppesg/paper28.pdf. | ||
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013. | |||
Tsao U, Zasloff HB, inventors; The Lummus Company, assignee. Production of ethylene from ethanol. US patent 4,134,926. January 16, 1979. | |||
[ | US Energy Information Administration. Today in Energy - Daily Prices [Internet]. US EIA; c2015 [cited 3 Mar 2015]. Available from: http://www.eia.gov/todayinenergy/prices.cfm. | ||
[ | Weddle N. ICIS Pricing: Ethylene (Europe) [Internet]. Reed Business Limited; c2015 [cited 10 Mar 2015]. Available from: http://www.icis.com/chemicals/ethylene/europe/ | ||
Zhang M, Yu Y. Dehydration of Ethanol to Ethylene. Industrial Engineering and Chemical Research. 2013; 52:9505-9514. | |||
[ | 190 Proof Ethanol: Technical Data Sheet [Internet]. Decon Labs Inc; c2012 [cited 13 Mar 2015]. Available from: http://www.deconlabs.com/tds/ETHANOL%20190%20%20PROOF.pdf. | ||
=Appendix I - Process Flow Diagram= | =Appendix I - Process Flow Diagram= | ||
[[File:PFD B1.JPG|1200px]] | [[File:PFD B1.JPG|1200px|center]] | ||
=Appendix II - HYSYS Simulation= | =Appendix II - HYSYS Simulation= | ||
[[File:HYSYS_B1.PNG|1200px]] | [[File:HYSYS_B1.PNG|center|1200px]] | ||
=Appendix III - Material Streams= | =Appendix III - Material Streams= | ||
| Line 74: | Line 149: | ||
[[File:Energy_B1.png|600px|center]] | [[File:Energy_B1.png|600px|center]] | ||
=Appendix V - Economics= | [[File:Utilities.PNG|600px|center]] | ||
=Appendix V - Equipment Sizing= | |||
[[File:Equipment sizes.png|center]] <br> | |||
=Appendix VI - Economics= | |||
[[File:Econsummary_B1.png|center]] <br> | [[File:Econsummary_B1.png|center]] <br> | ||
[[File:Capcost_B1.png|center]] <br> | [[File:Capcost_B1.png|center]] <br> | ||
Latest revision as of 16:29, 17 March 2015
Team B1 Final Report
Authors: Harry Poppick, Emm Fulk and Scott Smith
Instructors: Fengqi You, David Wegerer
March 13, 2015
Executive Summary
The following report outlines the design and analysis of a process converting ethanol to chemical-grade ethylene via catalytic dehydration. This process consumers 100,000 kg/hr of 95 wt% ethanol, 5 wt% water feedstock and produces 53,000 kg/hr of 99.7 wt% chemical-grade ethylene. The ethanol feed passes through two fixed-bed reactors, each packed with an aluminum oxide catalyst, and the resulting ethylene is purified by two flash tanks and by absorption with water. The process was modeled using Aspen HYSYS simulation software to estimate mass and energy flows and equipment sizing requirements. Based on results of this initial simulation, several alternatives are discussed for increasing the process efficiency and profitability. The use of a fluidized bed reactor or a more ethylene-selective catalyst could increase the production of ethylene per ethanol basis. Additionally, several methods of purification to more valuable polymer-grade ethylene are proposed.
The economic feasibility of this process was evaluated using Aspen Economic Analyzer with the Icarus economics package. Capital cost is approximately $16.4 million total with an operating cost of $465 million per year. The current market prices of the ethanol feed and ethylene sales price are $1.44/gallon and $1350/metric ton respectively. The cost of feed ethanol, process utilities and miscellaneous operational costs total to about $427 million/year. At full operation, revenue at current prices would be approximately $640 million per year. The predicted 10-year net present value of the process is approximately $391 million with an IRR of 79% and a payback period of 2.7 years. A sensitivity analysis indicates that the process is most sensitive to feed prices, product prices and operation cost.
Several recommendations are made for both cost reduction and profit maximization, including the design of a heat exchanger network and the optimization of fired heaters that require high capital investment and large amounts of fuel. A safety review of the process is recommended, as well as the minimization of flue gas effluent and wastewater. It is also suggested that the construction of an onsite wastewater treatment center be considered. Overall, the initial analysis indicates this to be a profitable process and it is recommended the project move forward.
Introduction
Ethylene is a critical chemical precursor to a number of different industrial products. It is often derived from petroleum sources and polymerized to polyethylene, one of the most ubiquitous plastics today. (American Chemistry Council, 2013) Recently there has been a significant effort to develop production pathways utilizing biologically-derived feedstocks, such as corn or cellulosic ethanol, rather than traditional fossil-fuel sources. Ethylene can be produced from bioethanol via catalytic dehydration over an aluminum oxide catalyst. The proposed process converts 100,000 kg/hr of 95 wt% ethanol, 5% water feed to 53,000 kg/hr of 99.7% chemical-grade ethylene when operating at full capacity. This process was found to be economically viable with a 10-year net present value (NPV) of $131 million (2014 USD) and a payback period of approximately 2.7 years. This memo will detail the proposed process, as well as outline potential alternatives and economic viability of the project.
Technical Approach
Many bioethanol plants are located in the Midwest where large amounts of feedstock crops, such as corn, are grown. As such, this plant will be located in Story county, Iowa, near a large-scale bioethanol production plant. (DuPont Nevada Site, 2012) It is assumed that the supply of bioethanol is consistent and readily available. It is also assumed that other reagents - namely, the aluminum oxide catalyst, heater fuel, utility water and freon refrigerant - are readily available, and that wastewater treatment facilities are located near enough to facilitate the off-site treatment of wastewater. Plant capacity was based on current mid-sized industrial ethylene plants. (Designing Ethylene Plants, 2014)
HYSYS simulations were performed with the NRTL fluid package and Peng-Robinson vapor model. The NRTL fluid package is an activity coefficient model that is especially useful to model the separations of alcohols in aqueous solutions. (Suppes, 2015) In addition, NRTL uses models of binary pairs of components, much like the Wilson equation, to predict chemical interactions at the molecular level. The NRTL package can thus be easily extended to ternary and higher order chemical systems, making it ideal for this type of simulation. The Peng-Robinson vapor model was selected as it is well-suited to account for the non-idealities of the vapor phase reactions and separations integral to this process. (ASPENtech, 2014)
It was assumed that the feed enters the process at ambient temperature and pressure (25 °C, 1 atm) and at a consistent rate and composition with negligible quantities of impurities. The ethanol feed was assumed to be 95% ethanol and 5% water by mass, which is the commercial standard for industrial ethanol. (190 Proof Ethanol, 2012) Storage vessels for the feed ethanol, waste streams and products are assumed to be located nearby to the process. An initial feed pump compensates for pressure drops through the process and reduces the duty on downstream compressors. (Hadawey, 2015)
Natural gas, assumed to consist of 83% methane, 16% ethane and 1% nitrogen by mass, was supplied to two fired heaters at a flow rate estimated by HYSYS. (Methane, 2015) Air fed to the process to enable combustion was assumed to be 23% oxygen and 77% nitrogen by mass. (Composition of Air, 2015) Air streams were fed at 50% molar excess in order to ensure complete combustion and prevent the formation of carbon monoxide in the heater exhaust. Total flue gases are assumed to be 230,000 kg/hr, or 2.2 million tons annually, and consist of 73.8% nitrogen gas, 10.5% carbon dioxide, 8.2% water vapor, and 7.5% oxygen by mass. The only compound of concern in these emissions is the carbon dioxide, a known greenhouse gas. At the time of this report, the quantities of carbon dioxide emitted by this plant annually are within acceptable EPA specifications. (Summary of the Clean Air Act, 2015)
Due to the difficulty of simulation equilibrium and conversion reactions in a single vessel in HYSYS, these two reaction types were simulated in separate equilibrium reactor and conversion reactor units in series. In addition, while HYSYS indicated that reactor units would output separate vapor and liquid streams, it was assumed that in practice this would take the form of a single, two phase stream between each unit. To account for this, mixers were installed downstream of each reactor to combine the liquid and vapor phases of the reactor effluent. The three consequent reactions considered are as follows:
The optimal reaction temperature was determined to be 400 °C, which promotes the equilibrium reaction of ethanol to ethylene while minimizing byproducts. (Zhang and Yu, 2013) While other byproducts may be produced in this process, these reactions are typically taken to comprise of the bulk of the dehydration reaction. Heat losses over these reactors were assumed to be negligible. (Zhang and Yu, 2013)All major process equipment is assumed to be constructed of high strength carbon steel. (AspenTech, 2012)
The “Sizing Utility” within the Icarus Economic Evaluation module was used to estimate sizes of major equipment based on the process simulation. These values can serve as a benchmark for an initial plant design. Icarus KbaseTM implements a wide variety of mechanical design standards, including ASME and BS5500, in order to calculate accurate equipment sizing. The Icarus sizing module combines equipment prices for standard chemical processing equipment as well as correlations and sizing factors. The advantage of using a software package such as HYSYS to price out individual equipment is that it utilizes up-to-date prices from vendors and distributors when estimating costs. A more in-depth overview of the correlations included in this software can be found in Towler and Sinnott (2009).
Process Flowsheet
The following process is based primarily on a 1983 US patent titled “Process for dehydration of a low molecular weight alcohol.” (Barrocas and Baratelli, 1983) The process flow diagram can be seen in Figure 1. A larger version of the PFD is included in Appendix I and the HYSYS simulation setup in Appendix II.
The feed enters the process at a rate of 100,000 kg/hr, a temperature and pressure of a 25 °C and 1 atm and at a composition of 95 wt% ethanol and 5 wt% water.It is initially pressurized to 4335 kPa by centrifugal pump P-101 and subsequently heated to 400 °C by fired heater F-101. The heated and pressurized feed enters fixed bed reactor R-101 and contacts with the aluminum oxide catalyst. At this point it dehydrates to form the product, ethylene, as well as the byproducts water, hydrogen, diethyl ether and acetaldehyde. Fired heater F-102 and reactor R-102 increase the overall conversion of the process. Both fired heaters F-101 and F-102 are fueled with natural gas and fed with excess air. The composition of the reactor train effluent is 54.5 wt% ethylene, 37.1 wt% water, 8.1 wt% unreacted ethanol and trace amounts of diethyl ether, acetaldehyde, and hydrogen contaminants.
The pressure of the reactor train effluent is increased to 4500 kPa by compressor K-102 and the temperature decreased to 100 °C by exchange with cold water in heat exchanger E-201. The stream then expands and flashes in flash vessel FL-201, which separates out 82.3% of the unreacted ethanol, 96.6% of the water and virtually all of the acetaldehyde contaminant in the bottoms while sending the ethylene-rich vapor phase out of the top. The top product of FL-202 is compressed to 3500 kPA by K-202 and cooled to 0.0 °C by a freon refrigerant in E-202. At this point the stream is flashed for a second time in vessel FL-202 to remove additional impurities. The top outlet stream consists of 99.5 wt% ethylene, which is fed to the bottom of a 10-tray absorption column that removes a fraction of the residual diethyl ether as well as the remainder of the unreacted ethanol. A stream of absorbent water is fed to the top of the column at 10,000 kg/hr. The product exits the top of the column at a rate of 54,600 kg/hr and has a purity of 99.7% ethylene. Diethyl ether makes up 0.2% of the remaining product stream and is the primary contaminant. Trace amounts of residual water and hydrogen are also present.
Full tables of material and energy streams can be found in Appendices III and IV respectively.
Process Alternatives
Reactor Alternatives
Two fixed bed reactors, alternating with fired heaters, are used in the dehydration reaction of ethanol to ethylene. However, a fluidized bed reactor can offer better mixing and temperature control than a fixed bed reactor and thus provide the highest possible conversion of ethanol with the greatest selectivity to ethylene. (Tsao and Zasloff, 1979) Fluidized bed reactors also provide a lower rate of catalyst coking and byproduct formation, minimizing separation and catalyst regeneration costs. (Morschbacker, 2009) The major downside of fluidized bed reactors is that few large-scale processes have been built since the technology was developed in the late 1970s. (Tsao and Zasloff, 1979; Morschbacker, 2009) Although implementation of fluidized bed reactors could potentially increase the purity of the ethylene product and/or decrease costs, a small scale pilot process would need to be built and tested before it would be possible to develop an industrial-scale facility.
Catalyst Alternatives
Aluminum oxide, specifically gamma alumina, is the most industrially-common catalyst and has been historically used due to its low cost and high stability. (Tsao and Zasloff, 1979; Fan and Dai, n.d.) Recent improvements in selectivity and catalyst stability of activated alumina have been achieved by incorporating titanium oxide into the catalyst, although this increases reaction temperature by approximately 50 °C and decreases catalyst lifespan. (Chen and Li, et. al. 2007) There is also significant ongoing research into HZSM-5 zeolite and heteropolyacids, which are being explored as potential alternatives to alumina oxide. A nanoscale HZSM-5 zeolite catalyst has been shown to operate at the relatively low temperature of 200-300°C, with a 100% ethanol conversion and 99.7% selectivity to ethylene. Initial research has also indicated that it maintains greater than 98% selectivity for 630 operational hours. (Fan and Dai, n.d.) However, the current low cost and ready availability of unmodified alumina oxide makes it a better choice for a process of this scale.
Purification Alternatives
As specified in this process, the reactor effluent is purified by two flash drums and absorption with water to produce chemical-grade ethylene. If chemical-grade ethylene is indeed the desired product, other purification options include caustic washing of the reactor effluent and water removal in a desiccant drying bed. (Morschbacker, 2009) While this purification train is relatively simple and does not require harsh chemical reagents, polymer grade ethylene is more commercially valuable and the additional cost of removing water and diethyl ether contaminants may be justified by increased revenue. Cryogenic distillation was initially simulated to produce polymer-grade ethylene, but utility requirements were found to render this separation economically unviable. (Barrocas and Baratelli, 1983) Further research and optimization may find this purification strategy to be a more valid process. Alternatively, the addition of a cryogenic turboexpander or the use of alternative adsorbents may increase product purity to polymer grade. (Jumonville, 2010) Initial HYSYS simulations indicate that these operations may be feasible with further refinement.
Wastewater Treatment
Due to the large volume of wastewater produced, building an on-site wastewater treatment facility may prove to be more economical than paying for third-party wastewater treatment. Flash vessel FL-201 produces 44,600 L/hr of wastewater contaminated with 18.7 vol% ethanol and 0.6 vol% acetaldehyde, while FL-202 produces 2940 L/hr of an aqueous waste consisting of 56.8 vol% ethanol, 42.0 vol% water and 0.9 vol% diethyl ether. The absorption column produces approximately 10,200 liters/hr of wastewater contaminated with 1.2 vol% ethanol, 0.2% diethyl ether and 0.2% acetaldehyde. Utilizing distillation to separate these streams into clean water and volatile organic waste could ultimately reduce the quantity of waste that requires disposal and reduce the overall costs of the process.
Process Equipment
Relevant sizing information for each unit operation, estimated by the method described in the “Technical Approach,” can be found in Appendix V. Equipment and piping is assumed to be constructed of carbon steel. It should be noted that the two fired heaters are proportionately large enough to heat the entirety of the process stream and are thus larger than standard equipment.
Economic Analysis
Economics Overview
All final values are normalized to June 2014 USD by CEPCI scaling. It is assumed that all products produced are sold at current market prices.
The Aspen Economic Evaluation module within HYSYS v8.0 was used to estimate capital and operating costs for a 10-year plant life span with a construction start date of April 2015. The price of the 95% ethanol feed stream was estimated at $1.44 per gallon, while the chemical grade ethylene produced is currently selling for approximately $1350 per metric ton. (Today in Energy, 2015; Weddle, 2014) Mapping and sizing calculations were performed by the economics module and a preliminary economic evaluation was completed. Equipment sizes, also estimated by HYSYS, were reviewed to ensure that height, diameter, and volume specifications were within realistic ranges.
The process was ideally projected to operate 360 days per year with a start-up time of one year. In addition, the tax rate was approximated at 40%, an interest rate at 20%, and a salvage value of 20%. Escalation percentages were compounded yearly for the product, raw material, and operating costs. The total capital cost for this process is approximately $16.4 million, which includes all equipment and piping described in the process. The most expensive equipment used in this process are the two fired heaters F-101 and F-102, which have an exceptionally large heat exchange area to adequately heat the feed. Standard heaters may not suffice and additional costs may be incurred for the raw materials and manufacture of this equipment. Including a feed preheater or additional fired heaters may reduce this cost in subsequent design iterations.
The total operating cost is approximately $465 million per year, about $427 million of which is the cost of the ethanol feed and natural gas for the two fired heaters. An additional $1.73 million per year is used for utilities including the cooling water and refrigerant. The labor and maintenance costs of this industrial process were also accounted for within the simulation’s economic analysis. The HYSYS simulation software, in conjunction with the Icarus economic analyzer, estimated there should be 5 operators per 8 hour shift with a cost per operator of $20 per hour. This results in a total operating labor cost of approximately $880,000 per year. In addition, the process will require one supervisor per shift with an hourly rate of $35 for total supervisor salaries of $307,000 per year. Finally, a fixed maintenance rate was estimated at $205,000 per 8000 hours of operation for a total cost of $225,000 per year.
Total product sales are roughly $640 million per year at full operation. The estimated income for this process is thus approximately $175 million per year. After ten years, the plant is projected to have a net present value (NPV) of $391 million and an internal rate of return of approximately 79%. In addition the payout period for the initial investment is estimated to be about 2.7 years. Figure 2 shows the projected cash flow for the project.
Sensitivity Analysis
A sensitivity analysis was performed to investigate the effects of fluctuating capital costs, operating costs, raw materials, utility costs and ethylene sales price on the 10-year net present value (NPV) of the project. This analysis was performed by scaling these cost factors according to typical ranges given in Towler and Sinnott and calculating the adjusting the NPV accordingly. The results, illustrated in Figure 3, indicate that the project value is most sensitive to product sales, raw materials costs, and operating costs. Ethylene and ethanol prices are market-determined and cannot be controlled for in the process design, so further optimization should be focused on minimizing the operations cost of the process. (Today in Energy, 2015; Weddle, 2014) However, in feed and product prices do still have a profound effect on profitability and feasibility. A more rigorous market analysis will better inform the profitability outlook.
Conclusions/Recommendations
The proposed process produces approximately 460,000 metric tons of 99.7 wt% ethylene annually, with a 10-year net present value of $131 million. If current economic conditions hold, it is recommended that this project proceed to implementation following further design improvements and optimization.
Future design iterations should focus on process optimization, particularly in minimizing operating costs as prescribed by the results of the sensitivity analysis. The development of a heat-exchanger network, potentially with Aspen Energy Analyzer, is one option for decreasing utility requirements and increasing the efficiency of the process overall. Optimization of the fired heaters could also result in decreased operational costs. Although overall profitability is less sensitive to capital costs, reviewing and adjusting equipment sizing would also improve the economic outlook. In particular, exploring alternative heating strategies at the beginning of the process could significantly decrease capital costs. Although there are not any particularly dangerous chemicals utilized in the process, an overall process review should also consider potential failure modes to minimize the safety risk to plant workers and nearby residents.
In addition to economic and safety considerations, harmful emissions and waste streams should be minimized both to bring down the cost of treatment and to decrease environmental effects. The two fired heaters consume a large amount of natural gas per year, emitting about 200,000 tons of CO2 annually, and account for more than half of the capital cost of the process. Although no known toxic gases are produced, carbon capture or other emissions-reduction methods should be considered. Additionally, enough wastewater is produced by this process that building an onsite wastewater treatment center should be considered. This could not only decrease operating costs by eliminating the need for an external contract but also give the plant more control over the final purity of the treated wastewater.
Finally, the current model assumes that all ethylene produced will be sold at a current prices and price or product sales will not be affected by economic variability. A more detailed study of the chemical-grade ethylene market, as well as a more thorough understanding of sales and distribution logistics, would increase the surety of a profitable process.
In summary, the proposed process yields chemical grade ethylene via the catalytic dehydration of ethanol. A 100,000 kg/hr of 95 wt% ethanol, 5% water feed is converted to 53,000 kg/hr of 99.7% chemical-grade ethylene. After 10 years of operation, including a one-year startup time, the process has an NPV of $391 million with an IRR of 79% and a payback period of 2.7 years. Pending further optimization, this process was determined to be viable and it is recommended for the project to move forward.
References
AspenTech. Appendix A - Property Methods and Calculations. Simulation Basics. 2014;A-3 - A-26.
AspenTech. Aspen HYSYS Help File v8.0. Aspen Technology, Inc. 2012.
Barrocas H, Baratelli F, inventors; Petroleo Brasileiro S.A., assignee. Process for dehydration of a low molecular weight alcohol. US patent 4,396,789 A. 1983 Aug 2.
Britannica.com. Methane (Chemical Compound) [Internet]. Encyclopædia Britannica Online; c2015 [cited 13 Mar 2015]. Available from: http://www.britannica.com/EBchecked/topic/378264/methane.
Chen G, Li S, Jiao F, Yuan Q. Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors. Catal Today. 2007;125:111–119.
Composition of Air [Internet]. Engineering Toolbox; n.d. [cited 13 Mar 2015]. Available from: http://www.engineeringtoolbox.com/air-composition-d_212.html.
Designing Ethylene Plants. Ethylene [Internet]. Technip; c2014 [cited 13 Mar 2015]. Available from: http://www.technip.com/en/our-business/onshore/ethylene.
DuPont Nevada Site Cellulosic Ethanol Facility [Internet]. DuPont Chemical; c2012-2015 [cited 11 Mar 2015]. Available from: http://biofuels.dupont.com/cellulosic-ethanol/nevada-site-ce-facility/.
Epa.gov. Summary of the Clean Air Act, 2015 [Internet]. United States Environmental Protection Agency; c2015 [cited 13 Mar. 2015]. Available from: http://www2.epa.gov/laws-regulations/summary-clean-air-act.
Fan D, Dai DJ, Wu HS. Ethylene Formation by Catalytic Dehydration of Ethanol with Industrial Considerations. Materials. 2012 Dec;6:101-115.
Jumonville, J. Tutorial on Cryogenic Turboexpanders. Proceedings of the Thirty-Ninth Turbomachinery Symposium; 2010 Oct 4-7; Houston, TX. Houston: Texas A&M University; 2010.
Morschbacker, A. Bio-Ethanol Based Ethylene. Polymer Reviews. 2009; 49:79-84.
Statista.com. Production of chemicals and plastics in the U.S. in 2013, by type (in 1,000 metric tons) [Internet]. American Chemistry Council; c2014 [cited 15 Jan 2015]. Available from: http://www.statista.com/statistics/299725/total-us-plastics-and-chemicals-shipments-by-type/.
Suppes GJ. Selecting Thermodynamic Models for Process Simulation of Organic VLE and LLE Systems [Internet]. The University of Missouri-Columbia, Dept of Chemical Engineering; c2002 [cited 13 Mar 2015]. Available from: http://web.missouri.edu/~suppesg/paper28.pdf.
Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.
Tsao U, Zasloff HB, inventors; The Lummus Company, assignee. Production of ethylene from ethanol. US patent 4,134,926. January 16, 1979.
US Energy Information Administration. Today in Energy - Daily Prices [Internet]. US EIA; c2015 [cited 3 Mar 2015]. Available from: http://www.eia.gov/todayinenergy/prices.cfm.
Weddle N. ICIS Pricing: Ethylene (Europe) [Internet]. Reed Business Limited; c2015 [cited 10 Mar 2015]. Available from: http://www.icis.com/chemicals/ethylene/europe/
Zhang M, Yu Y. Dehydration of Ethanol to Ethylene. Industrial Engineering and Chemical Research. 2013; 52:9505-9514.
190 Proof Ethanol: Technical Data Sheet [Internet]. Decon Labs Inc; c2012 [cited 13 Mar 2015]. Available from: http://www.deconlabs.com/tds/ETHANOL%20190%20%20PROOF.pdf.
Appendix I - Process Flow Diagram
Appendix II - HYSYS Simulation
Appendix III - Material Streams
Appendix IV - Energy Table
Appendix V - Equipment Sizing
Appendix VI - Economics