Site condition and design: Difference between revisions
| (105 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
<br> | |||
Authors: Alex Chandel, Eric Jiang, Minwook Kim, Todor Kukushliev, William Lassman | Authors: | ||
Alex Chandel, Eric Jiang, Minwook Kim, Todor Kukushliev, William Lassman (ChE 352 in Winter 2014) | |||
Sheridan Lichtor (ChBE 352 in Winter 2016) | |||
Date | Steward: Daniel Garcia, David Chen, Fengqi You | ||
Date Presented: 2/21/2016 & 1/17/2014 | |||
<br> | |||
== Introduction == | == Introduction == | ||
One of the first and most important decisions when designing a chemical plant is plant location. If a new plant is being built, a suitable site must be found and a plant layout considered. However, if the chemical plant is being built on the site of an old plant (possible upgrade or expansion) the existing site’s infrastructure must be considered. Of incredible importance are local laws and ordinances concerning chemical disposal, safety of the local population, and considerations for the employed operators. | |||
Suitable locations for chemical plants often have several plants in close proximity. The existence of these locations is often beneficial as there are often living infrastructure nearby to support the labor. Figure 1 shows the distribution of labor across the US and implicitly the common locations of many chemical plants. | |||
[[File:Map of ChE.png|frame|center|border|<div align=center> Figure 1: Employment of Chemical Engineers by Area as of May 2012 <div>]] | |||
<div align=left> | |||
Above is shown the occupational employment density of chemical engineers separated county. It is noticeable that the coastal areas of the United States are most attractive for chemical process industries due, no doubt, to the easy access to water transportation routes, which are cheaper and faster than land transportation. Building a process plant in any of the “240-3,740” density shaded regions would capture the additional benefit of having the process plant built in an area where supporting industries already thrive, therefore making repairs and operational costs as a whole as low as possible as determined by location. The states of greatest chemical process plant density are California, Arizona, Texas, Louisiana, Mississippi, Illinois, Michigan, Indiana, Ohio, and the majority of the east coast states. The local corporate tax rates of these locations are highly dependent on income bracket as per the information found at http://www.taxadmin.org/fta/rate/corp_inc.pdf | |||
== Geographical selection == | == Geographical selection == | ||
| Line 15: | Line 27: | ||
=== Factors considered === | === Factors considered === | ||
==== Natural resources ==== | ==== Natural resources ==== | ||
Transporting materials to and from the plant is a huge operational cost that is heavily factored into when selecting a plant location. Therefore, choosing to build a plant near natural resources reduce the operational cost of the plant tremendously. Natural resources such as river, lake, sea, and oil well near operating plants can be a huge bonanza for them. | |||
Major chemical plants processes need cooling system, which require immense amount of water. If river, lake, or sea is in close proximity, plants can utilize the water readily and relatively cheap. Plant needing of a great energy may build a dam on a river to resolve power issue. Upstream oil sectors look for oil wells to operate and drill out the oil and gas. Companies find themselves saving or making money when they build a plant near natural resources which they can take advantage. | |||
==== Weather ==== | ==== Weather ==== | ||
Local climate conditions should be a strong consideration in the selection of a site for (chemical) processing or production facilities. As previously discussed, a variety of considerations are involved in site selection, including: the availability of raw materials, transportation capability, availability of labor, waste capacity and regulations, and local community and environmental considerations. In some instances, the aforementioned considerations will be prioritized over considerations regarding the local climate of the site; in other instances, a company is limited to the geographic locations in which they already own land or are involved in manufacturing and production. In the case where a company cannot select a site whose climate is optimized to meet production needs, there are several design considerations that need to be taken into account to accommodate the local climate conditions when setting up a facility. | |||
A reality of large chemical processing and production facilities is that it is oftentimes difficult to control the ambient environmental conditions in which manufacturing occurs. In industry, it is common to use open, structural steelwork buildings to house processing equipment (Towler 511). Oftentimes, this type of setup provides little protection from the weather and local climate. In some cases closed buildings house processing equipment in operations that can be particularly sensitive to disturbances (such as the disturbances that adverse weather conditions might present), in small plants, or in processes that have ventilation components for which the vent gas scrubbing is necessary (Towler 511). It is generally cheaper, however, to use open setups for production given their lower capital costs of construction. | |||
The usage of open setups also has downsides, particularly with respect to local climate. A site in which adverse climate conditions are commonplace will logically result in higher costs for the setup and upkeep of the facility. Examples of climate conditions that should be factored into the design process for plant location and site selection include: temperature, humidity, wind, precipitation, and natural disaster frequency (hurricanes, tornadoes, earthquakes, tsunamis). Strong, reinforced structures are required in locations that are subjected to high winds and in climates that receive hurricanes, tornadoes, earthquakes, and tsunamis (Towler 507). This section will specifically focus in depth on the implications of two critical climate conditions, temperature and humidity, as they relate to chemical processing and site selection. | |||
===== Temperature ===== | |||
In many geographic locations, temperature can fluctuate significantly depending on the time of year. In these cases, processing equipment should be able to withstand the stresses of gradual annual shifts in temperature, as well as faster day-to-day changes. In areas where the climate crosses 0 ºC, cycles of freezing and thawing may weaken the structural integrity of pipes and other processing equipments. Abnormally low temperatures may necessitate the addition of heating and added insulation, whereas abnormally high temperatures may require the provision of additional cooling systems to control the process temperature (Booth 154). Furthermore, the potential for a catastrophic burst or leakage is possible in cases where freezing water has the possibility of touching or interacting with pipelines or processing equipment. Specifically, in some circumstances a valve or joint might have a defect or crack that could propagate and cause a catastrophic failure from the constant freezing and thawing cycles on the equipment (Booth 154). | |||
Extreme temperatures are known to lower productivity of laborers and machinery. Heat, for example, can impact machinery that uses belts; warm temperatures loosen belts and can lower the product output due to processing irregularities stemming from belt slippage (Booth 157). Another general concern with temperature is that worker labor and productivity is adversely affected by extreme cold and hot; this may occur either in instances where production is not shielded from extreme outside climates or when production itself necessitates extreme temperature climates. Extreme heat, in particular, can hinder the mental and physical capability of workers; as a result, many companies give workers enforced vacation and additional mandatory break times. While this is good for the health and safety of the workers, it is also at the company’s expense. Local climate temperature should not be overlooked in the site selection process for a chemical plant. | |||
Figure 2 demonstrates the drastic differences that can exist in minimum and maximum temperature for one location (Chicago, IL) based on seasonality. According the graph, the minimum and maximum temperature over the year 2015 in Chicago fluctuates on average 11 ºC within each month ("World Weather"). Furthermore, the average temperature in Chicago during July is 23 ºC and the average temperature in January is -6 ºC; this is a 29 ºC range in the average temeperature throughout the year; the equipment used in a chemical plant, and also the materials being processed, need to withstand this large annual fluctuatiaon in temperature. | |||
[[File:Chicago Temperature.png|frame|center|border|<div align=center> Figure 2: Minimum and Maximum Temperature Throughout the Year in Chicago <div>]] | |||
<div align=left> | |||
===== Humidity ===== | |||
Like temperature, humidity can fluctuate significantly depending on the season and even time of day. Unlike temperature, however, humidity is less so a problem for processing equipment as it is for the chemicals and substances being processed. Namely, hygroscopic effects become significant factors associated with high humidity processing environments (Booth 156). Hygroscopy concerns itself with a material’s affinity to pull in and store moisture from the environment, either via absorption or adsorption. Moisture uptake and hygroscopic effects are a major problem in cases where knowing the weight fractions of different materials is critical. For example, reactions usually call for specific amounts and weight fractions of reactants in order to get the desired product and meet detailed specifications. If one is not aware of the water fraction of the materials going into the reaction, then there may be unforeseen (and potentially very dangerous) consequences associated with either having an incorrect weight fraction reactant entering the reactor or having water involved in the reaction. | |||
Powders are also very susceptible to hygroscopic effects. Many food products, such as baked goods, use powder ingredients that are sensitive to moisture effects; moisture content of packaged foods is critical to shelf life and preventing the growth of bacteria. Outside of food applications, powders are also used in making glass, composites, ceramics, and pharmacological drugs. In their processing, it is critical to prevent caking by limiting the moisture uptake. This can impact the rheology (flow behavior) of the materials, which ultimately will have implications on the quality and purity of the final product. By contrast, some materials, such as cotton, linen, jute, and hemp, need to spun and woven in humid environments. Examples of hygroscopic materials include: glucose, flour, starch, glycerin, calcium chloride, and sodium chloride (Booth 157). | |||
A psychrometric diagram, such as the one in Figure 3, can explain the correlation between dry-bulb temperature, wet-bulb temperature, and relative humidity (NSF). Dry-bulb temperature is simply the ambient air temperature and is unaffected by the moisture content of the air; dry-bulb temperature is measured by a standard thermometer (NSF). Wet-bulb temperature is the saturation temperature of air, and it is the lowest temperature at which water can evaporate into the air; this is measured using a thermometer with a moist cloth wrapped around the bulb (Padfield). Relative humidity is the amount of water vapor present in air, and is calculated as the ratio of partial pressure of water vapor to the equilibrium vapor pressure of water (Padfield). Figure 3 suggests strong correlations between air temperature moisture content in the air, measured as relative humidity. For example, at constant dry-bulb temperatures, the relative humidity increases as the wet-bulb temperature increases. Likewise, at constant wet-bulb temperatures, the relative humidity decreases as the dry-bulb temperature increases. In selecting the location of a chemical processing facility, especially for facilities where there is little control over the ambient temperature, it is important to understand that small changes in temperature can have exponential consequences on the moisture content/ relative humidity of the air; these temperature and humidity changes can affect both processing equipment and the materials being processed. | |||
[[File:RH Graph.jpg|frame|center|border|<div align=center> Figure 3: Relative Humidity as a Function of Wet-Bulb and Dry-Bulb Temperatures <div>]] | |||
<div align=left> | |||
===== Weather Example ===== | |||
A variety of case studies have looked at weather effects on chemical processing. One such case explored the effects of temperature and humidity on phenol-formaldehyde resin bonding (Wang 253). Phenol-formaldehyde resin is a thermosetting adhesive that polymerizes and reacts with wood as part of the curing process in wood composite manufacturing. The strength of the resin bond is thought to be influenced by a variety of factors related to processing environment, including temperature and humidity. Figure 4 depicts the results from a study that compared the bond strength as a function of temperature, relative humidity, and bonding time (Wang 258-259). | |||
[[File:RH & Temp Effects.jpg|frame|center|border|<div align=center> Figure 4: Effects of Humidity, Temperature, and Bonding Time on Bond Strength <div>]] | |||
<div align=left> | |||
As the results suggest, drastically different resin strength profiles are expected depending on relative humidity. Considering just the samples that were bonded at 110 ºC, the resins that were cured at 41% relative humidity overall cured stronger than their counterparts that were cured at the same time but at higher relative humidities. An interesting feature that is prevalent in the 110 ºC bonding samples is that processing conditions at higher relative humidities is not always indicative of a depreciated bond strength. As the graph suggests between the 75% and 90% relative humidity samples, when bonded for less than 10 minutes, the 90% relative humidity samples actually are stronger than the 75% samples. However, after about 10 minutes the trend exists such that samples cured at higher relative humidities overall bond more weakly. | |||
There also appear to be striking differences between the 110 ºC and 120 ºC samples. In fact, it appears that less bonding time is required for the 120 ºC as is the time required to get comparable strengths for the 110 ºC samples. Also, at higher processing temperature of 120 ºC, it is evident that the samples at 75% relative humidity now can have comparable bond strengths as the samples at 41% relative humidity. Additionally, the samples at the 90% relative humidity also have somewhat higher binding strengths for the 120 ºC bonding temperatures compared to the 110 ºC bonding temperatures. | |||
Thus, this study indicates the appreciable differences that can exist in the product quality based on humidity and temperature effects. Thus, depending on the desired product qualities (bond strength in this resin study), humidity and temperature are critical metrics in defining the process environment. This phenol-formaldehyde resin study is particularly useful in demonstrating the effects of ambient relative humidity on the mechanical strength of the product, and relative humidity is definitely a parameter that could fluctuate depending on the weather patterns of the processing environment. Additionally, 10 ºC (the difference between bonding at 110 ºC and 120 ºC) is well within the monthly and seasonal temperature fluctuations of different locations; whether or not the weather could be attributed to such processing differences at these high temperatures is a possibility. | |||
==== Proximity to related chemical operations ==== | ==== Proximity to related chemical operations ==== | ||
The availability and price of raw materials for feed streams often play a large part in determining the plant location. For example, many ethylene plants are built in the Middle East near supplies of natural gas. | |||
If building a plant near raw materials is not possible, often the next determining factor is ease of transportation. For most chemicals, proximity to major road, rail, waterway, or ports are desired. For high-value products such as pharmaceuticals, proximity to air ports can be used to prevent degradation of product during transport. Ease of transportation results in cheaper logistics cost for transport between both suppliers and buyers. | |||
Proximity to utilities are important in chemical process. Water is ubiquitous in chemical plants and are often require in substantial amounts. Construction of plants near rivers and lakes are often desired to reduce the cost of process water. If drawing from local water is not possible, cooling towers will need to be used. Electrical power is required in all plants, often requiring plants to be built on available power grids. | |||
==== Laws and regulations ==== | ==== Laws and regulations ==== | ||
Federal laws will be listed as it serves as a baseline for the entire country. State and local laws sometimes are stricter than the established federal laws resulting. Property costs, property taxes, corporate income taxes, and fines also vary between states. Therefore, further consultation of the state and local laws must also be done beyond the laws listed in this text to ensure adherence to all laws required for the location of the plant. Below are several hallmark federal laws which proper treatment and disposal of waste in the air, ground, and water (Towler and Sinnott, 2013). | |||
===== The Clean Air Act ===== | |||
The Clean Air Act (CAA) was first passed in 1970 and was amended in 1990. The CAA empowers the EPA to set National Ambient Air Quality Standards (NAAQS)for the following seven contaminants: | |||
::*Ozone | |||
::*Carbon Monozide | |||
::*Lead | |||
::*Nitrogen Dioxide | |||
::*Sulfur Dioxide | |||
::*PM10: Partulate matter with mean diameter less than 10 μm. | |||
::*PM2.5: Partulate matter with mean diameter less than 2.5 μm. | |||
In addition to these seven contaminatns, the CAA also empowered EPA to regulate an additional 189 hazardous air pollutants listed in the National Emission Standards for Hazardous Air Pollutants. Failure to meet NAAQS levels will result in the requirement of remediation steps to be taken to lower emissions before the plant is allowed to be operational. | |||
===== The Clean Water Act ===== | |||
The Clean Water Act was first passed in 1972 and was amended in 1977 and 1987. It seeks to achieve clean water for swimming, boating, and protecting fish and water life. This act mandates that a permit from the EPA must be issued for discharge of pollutants into navigable waters. | |||
===== The Safe Drinking Water Act ===== | |||
The Safe Drinking Water Act was passed in 1974. It empowers the EPA to set standards on the required purity of any water which could be used for drinking. | |||
===== The Resource Conservation and Recovery Act ===== | |||
The Resource Conservation and Recovery Act was passed in 1976 to protect groundwater from contamination. This Act states that all waste producers are legally liable at any time from waste production to final disposal. Hazardous waste must be clearly labeled and tracked in transport and treated to levels specified by the EPA. | |||
==== Waste Minimization and Management ==== | ==== Waste Minimization and Management ==== | ||
===== Waste Minimization ===== | ===== Waste Minimization ===== | ||
Production of waste is arises naturally in any plant and require a noticeable amount of resources to take care of. Before even considering methods of managing ways, cost can significantly be reduced by efficient management by source reduction. Below are some source reduction strategies which can be employed: | Production of waste is arises naturally in any plant and require a noticeable amount of resources to take care of. Before even considering methods of managing ways, cost can significantly be reduced by efficient management by source reduction. Below is a five-step review often conducted to minimize waste production (Towler and Sinnott, 2013): | ||
::1. Identify waste: Identify what waste products are produced. | |||
::2. Economical Impact: Determine the size of the waste stream and the cost of treatment | |||
::3. Root causes: Determine the root cause of the waste streams. | |||
::4. Modifications: Analyze the effectiveness and cost of potential solutions. | |||
::5. Implement: Implement and optimize solutions of waste management. | |||
Below are some source reduction strategies which can be employed (Towler and Sinnott, 2013): | |||
::* Purification of feeds: Impurities in feed streams can lead to side reactions and formation of waste. Either purchase of purer feeds or employment of purification techniques which do not generate more waste can be used. Purification of feeds will also lead to the reduction of purge and vent streams. | |||
::* Protect catalysis and adsorbents: Catalysts are sensitive to containment in the feed and be deactivated. One method of protecting the catalyst or adsorbent is by employing a guard bed of material to absorb or filter out contaminants. | |||
::* Eliminate use of extraneous materials: Limiting the diversity of solvents is beneficial. The mixing of different solvents can result in waste formation when solvents are degraded. | |||
::* Increase recovery from separations: Higher product recovery results in lower concentrations of products in the the waste streams and less waste formation. | |||
::* Improve fuel quality: Cleaner-burning fuel can have less harmful emissions. | |||
::* Recycle or sell side-products: Rather than processing side-products, attempt to identify companies which might use the waste as raw material for another process. | |||
Keep in mind for all the strategies which can be employed to minimize waste production and therefore waste treatment, the overall cost must be considered. The savings from minimizing waste must be more than the additional cost implementing minimization. | |||
===== Waste Management ===== | ===== Waste Management ===== | ||
There are many methods of waste treatment and safe disposal. The availability and efficiency of these methods depend heavily on location. Adherence to federal, state, and local laws may further restrict the availability, of some of these techniques. Common techniques include: | |||
::* Dilution and dispersion. | |||
::* Discharge into municipal sewer. | |||
::* Physical treatments: absorption, adsorption. | |||
::* Chemical treatment: precipitation, neutralization. | |||
::* Biological treatment: composting, anaerobic digestion. | |||
::* Incineration. | |||
::* Landfill at controlled sites. | |||
::* Sea dumping. | |||
== Economical definition of cost == | |||
| Line 55: | Line 159: | ||
Property prices, rental fees, taxes, and existing company property in the area contribute to recurring investment costs. | Property prices, rental fees, taxes, and existing company property in the area contribute to recurring investment costs. | ||
== Site Layout: Design and Construction == | |||
== Site Design and Construction == | |||
=== Site Layout === | === Site Layout === | ||
The needs of a site for a chemical process vary considerably from process to process. In general, however, all chemical plants require the following: | The needs of a site for a chemical process vary considerably from process to process. In general, however, all chemical plants require the following (Towler and Sinnott, 2013): | ||
::*Shipping and receiving of products and raw materials | ::*Shipping and receiving of products and raw materials | ||
| Line 77: | Line 170: | ||
::*Offices and laboratories for management and quality control personnel | ::*Offices and laboratories for management and quality control personnel | ||
::*Medical and fire services for emergency management | ::*Medical and fire services for emergency management | ||
::*Cafeterias, parking lots, and other amenities for employees. | ::*Cafeterias, parking lots, and other amenities for employees. | ||
These auxiliary buildings are often referred to as ancillary structures and they are placed within a chemical process to minimize transportation of goods and personnel, and to maximize safety. The following procedure is followed when determining the site layout of a chemical process: | These auxiliary buildings are often referred to as ancillary structures and they are placed within a chemical process to minimize transportation of goods and personnel, and to maximize safety. The following procedure is followed when determining the site layout of a chemical process (Mecklenburgh, 1985): | ||
::1. Major process equipment is placed in a logical order to minimize transportation of process streams. Extra emphasis is placed on the separation and treatment of hazardous materials as quickly as possible. | ::1. Major process equipment is placed in a logical order to minimize transportation of process streams. Extra emphasis is placed on the separation and treatment of hazardous materials as quickly as possible. | ||
| Line 96: | Line 188: | ||
::7. Walkways and roadways are added as needed to assist with construction and transportation during plant operation. | ::7. Walkways and roadways are added as needed to assist with construction and transportation during plant operation. | ||
=== Site Construction === | === Site Construction === | ||
Site construction, along with process design, is an iterative process that follows a multi-step procedure (Mecklenburgh, 1985). | |||
Site construction, along with process design, is an iterative process that follows a multi-step procedure. | |||
==== Stage One Layout ==== | ==== Stage One Layout ==== | ||
| Line 123: | Line 212: | ||
The first step in constructing the plant is remediation and preparation of the land for construction of a chemical plant. This can include clearing the land of trees and vegetation, removing other natural obstacles such as boulders and ditches, implementing a drainage system, landscaping, grading to remove difficult topography, and anything else that is necessary. Site selection attempts to minimize costs associated with this step, but there is invariably some form of preparation required for every site. | The first step in constructing the plant is remediation and preparation of the land for construction of a chemical plant. This can include clearing the land of trees and vegetation, removing other natural obstacles such as boulders and ditches, implementing a drainage system, landscaping, grading to remove difficult topography, and anything else that is necessary. Site selection attempts to minimize costs associated with this step, but there is invariably some form of preparation required for every site. | ||
The second step is to construct all roadways, sidewalks, and fences required for both plant operation and plant construction. Costs associated with this step can range from 2 to 10 percent of the total capital investment for a chemical plant ( | The second step is to construct all roadways, sidewalks, and fences required for both plant operation and plant construction. Costs associated with this step can range from 2 to 10 percent of the total capital investment for a chemical plant (Peters et al., 2002). | ||
Process equipment and buildings are then constructed as soon as they are available. While construction schedules vary considerably from process to process, in some cases it is possible to perform the final construction steps once the process has already begun to operate, and the construction schedule is designed with this in mind (Mecklenburgh, 1985). | |||
A new aspect of construction of process equipment is a modular approach, where process equipment is assembled as completely as possible by the manufacturer and shipped while assembled. The advantage to this approach is a more comprehensive testing of the equipment by the manufacturer and less installation time once the equipment has arrived on site (Towler and Sinnott, 2013). | |||
== References == | |||
Booth WM. Transactions of the American Institute of Chemical Engineers. Volume VI. New York: D Van Nostrond Co; 1913. | |||
Mecklenburgh JC. Process plant layout. New York: Halsted Press; 1985. | |||
National Science Foundation. "Hands-on Activity: Swamp Cooler." Teach Engineering. College of Engineering at University of Colorado Boulder and Integrated Teaching and Learning, 19 Feb. 2016. Web. 21 Feb. 2016. <https://www.teachengineering.org/view_activity.php?url=collection/cub_/activities/cub_housing/cub_housing_lesson01_activity2.xml>. | |||
Padfield, Tim. "Glossary of the Microclimate Variables and Units Used in Conservation Physics." Conservation Physics. National Museum of Denmark, 14 June 2012. Web. 21 Feb. 2016. <http://www.conservationphysics.org/cpw/Storage/Fundamentals>. | |||
Peters MS, Timmerhaus KD, West RE. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw-Hill; 2002. | |||
Towler G, Sinnott R. General Site Considerations. In: Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013. | |||
Wang, Xiang-Ming, Bernard Riedl, Alfred Christiansen, and W. Geimer. "The Effects of Temperature and Humidity on Phenol-formaldehyde Resin Bonding." Wood Science and Technology 29.4 (1995): 253-66. Web. | |||
"World Weather & Climate Information." Weather and Climate: Chicago, United States of America. World Weather and Climate Information, 2015. Web. 20 Feb. 2016. <https://weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine,Chicago,United-States-of-America>. | |||
== External links== | == External links== | ||
* | * [https://en.wikipedia.org/wiki/Chemical_plant_cost_indexes Wikipedia - Chemical plant cost indexes] | ||
* [https://en.wikipedia.org/wiki/Chemical_plant#Clustering_of_Commodity_Chemical_Plants Wikipedia - Clustering of Commodity Chemical Plants] | |||
Latest revision as of 02:09, 22 February 2016
Authors: Alex Chandel, Eric Jiang, Minwook Kim, Todor Kukushliev, William Lassman (ChE 352 in Winter 2014)
Sheridan Lichtor (ChBE 352 in Winter 2016)
Steward: Daniel Garcia, David Chen, Fengqi You
Date Presented: 2/21/2016 & 1/17/2014
Introduction
One of the first and most important decisions when designing a chemical plant is plant location. If a new plant is being built, a suitable site must be found and a plant layout considered. However, if the chemical plant is being built on the site of an old plant (possible upgrade or expansion) the existing site’s infrastructure must be considered. Of incredible importance are local laws and ordinances concerning chemical disposal, safety of the local population, and considerations for the employed operators.
Suitable locations for chemical plants often have several plants in close proximity. The existence of these locations is often beneficial as there are often living infrastructure nearby to support the labor. Figure 1 shows the distribution of labor across the US and implicitly the common locations of many chemical plants.

Above is shown the occupational employment density of chemical engineers separated county. It is noticeable that the coastal areas of the United States are most attractive for chemical process industries due, no doubt, to the easy access to water transportation routes, which are cheaper and faster than land transportation. Building a process plant in any of the “240-3,740” density shaded regions would capture the additional benefit of having the process plant built in an area where supporting industries already thrive, therefore making repairs and operational costs as a whole as low as possible as determined by location. The states of greatest chemical process plant density are California, Arizona, Texas, Louisiana, Mississippi, Illinois, Michigan, Indiana, Ohio, and the majority of the east coast states. The local corporate tax rates of these locations are highly dependent on income bracket as per the information found at http://www.taxadmin.org/fta/rate/corp_inc.pdf
Geographical selection
The location surrounding a chemical plant can substantially influence its construction costs and operating costs, and may affect long-term profitability. Thus it is important to choose an appropriate location for every facility.
Factors considered
Natural resources
Transporting materials to and from the plant is a huge operational cost that is heavily factored into when selecting a plant location. Therefore, choosing to build a plant near natural resources reduce the operational cost of the plant tremendously. Natural resources such as river, lake, sea, and oil well near operating plants can be a huge bonanza for them.
Major chemical plants processes need cooling system, which require immense amount of water. If river, lake, or sea is in close proximity, plants can utilize the water readily and relatively cheap. Plant needing of a great energy may build a dam on a river to resolve power issue. Upstream oil sectors look for oil wells to operate and drill out the oil and gas. Companies find themselves saving or making money when they build a plant near natural resources which they can take advantage.
Weather
Local climate conditions should be a strong consideration in the selection of a site for (chemical) processing or production facilities. As previously discussed, a variety of considerations are involved in site selection, including: the availability of raw materials, transportation capability, availability of labor, waste capacity and regulations, and local community and environmental considerations. In some instances, the aforementioned considerations will be prioritized over considerations regarding the local climate of the site; in other instances, a company is limited to the geographic locations in which they already own land or are involved in manufacturing and production. In the case where a company cannot select a site whose climate is optimized to meet production needs, there are several design considerations that need to be taken into account to accommodate the local climate conditions when setting up a facility.
A reality of large chemical processing and production facilities is that it is oftentimes difficult to control the ambient environmental conditions in which manufacturing occurs. In industry, it is common to use open, structural steelwork buildings to house processing equipment (Towler 511). Oftentimes, this type of setup provides little protection from the weather and local climate. In some cases closed buildings house processing equipment in operations that can be particularly sensitive to disturbances (such as the disturbances that adverse weather conditions might present), in small plants, or in processes that have ventilation components for which the vent gas scrubbing is necessary (Towler 511). It is generally cheaper, however, to use open setups for production given their lower capital costs of construction.
The usage of open setups also has downsides, particularly with respect to local climate. A site in which adverse climate conditions are commonplace will logically result in higher costs for the setup and upkeep of the facility. Examples of climate conditions that should be factored into the design process for plant location and site selection include: temperature, humidity, wind, precipitation, and natural disaster frequency (hurricanes, tornadoes, earthquakes, tsunamis). Strong, reinforced structures are required in locations that are subjected to high winds and in climates that receive hurricanes, tornadoes, earthquakes, and tsunamis (Towler 507). This section will specifically focus in depth on the implications of two critical climate conditions, temperature and humidity, as they relate to chemical processing and site selection.
Temperature
In many geographic locations, temperature can fluctuate significantly depending on the time of year. In these cases, processing equipment should be able to withstand the stresses of gradual annual shifts in temperature, as well as faster day-to-day changes. In areas where the climate crosses 0 ºC, cycles of freezing and thawing may weaken the structural integrity of pipes and other processing equipments. Abnormally low temperatures may necessitate the addition of heating and added insulation, whereas abnormally high temperatures may require the provision of additional cooling systems to control the process temperature (Booth 154). Furthermore, the potential for a catastrophic burst or leakage is possible in cases where freezing water has the possibility of touching or interacting with pipelines or processing equipment. Specifically, in some circumstances a valve or joint might have a defect or crack that could propagate and cause a catastrophic failure from the constant freezing and thawing cycles on the equipment (Booth 154).
Extreme temperatures are known to lower productivity of laborers and machinery. Heat, for example, can impact machinery that uses belts; warm temperatures loosen belts and can lower the product output due to processing irregularities stemming from belt slippage (Booth 157). Another general concern with temperature is that worker labor and productivity is adversely affected by extreme cold and hot; this may occur either in instances where production is not shielded from extreme outside climates or when production itself necessitates extreme temperature climates. Extreme heat, in particular, can hinder the mental and physical capability of workers; as a result, many companies give workers enforced vacation and additional mandatory break times. While this is good for the health and safety of the workers, it is also at the company’s expense. Local climate temperature should not be overlooked in the site selection process for a chemical plant.
Figure 2 demonstrates the drastic differences that can exist in minimum and maximum temperature for one location (Chicago, IL) based on seasonality. According the graph, the minimum and maximum temperature over the year 2015 in Chicago fluctuates on average 11 ºC within each month ("World Weather"). Furthermore, the average temperature in Chicago during July is 23 ºC and the average temperature in January is -6 ºC; this is a 29 ºC range in the average temeperature throughout the year; the equipment used in a chemical plant, and also the materials being processed, need to withstand this large annual fluctuatiaon in temperature.
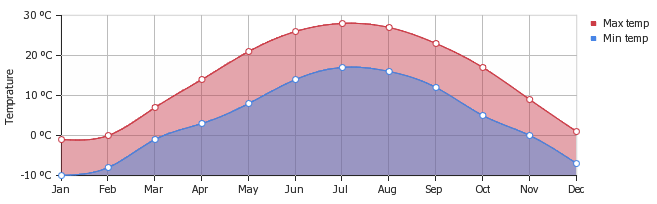
Humidity
Like temperature, humidity can fluctuate significantly depending on the season and even time of day. Unlike temperature, however, humidity is less so a problem for processing equipment as it is for the chemicals and substances being processed. Namely, hygroscopic effects become significant factors associated with high humidity processing environments (Booth 156). Hygroscopy concerns itself with a material’s affinity to pull in and store moisture from the environment, either via absorption or adsorption. Moisture uptake and hygroscopic effects are a major problem in cases where knowing the weight fractions of different materials is critical. For example, reactions usually call for specific amounts and weight fractions of reactants in order to get the desired product and meet detailed specifications. If one is not aware of the water fraction of the materials going into the reaction, then there may be unforeseen (and potentially very dangerous) consequences associated with either having an incorrect weight fraction reactant entering the reactor or having water involved in the reaction.
Powders are also very susceptible to hygroscopic effects. Many food products, such as baked goods, use powder ingredients that are sensitive to moisture effects; moisture content of packaged foods is critical to shelf life and preventing the growth of bacteria. Outside of food applications, powders are also used in making glass, composites, ceramics, and pharmacological drugs. In their processing, it is critical to prevent caking by limiting the moisture uptake. This can impact the rheology (flow behavior) of the materials, which ultimately will have implications on the quality and purity of the final product. By contrast, some materials, such as cotton, linen, jute, and hemp, need to spun and woven in humid environments. Examples of hygroscopic materials include: glucose, flour, starch, glycerin, calcium chloride, and sodium chloride (Booth 157).
A psychrometric diagram, such as the one in Figure 3, can explain the correlation between dry-bulb temperature, wet-bulb temperature, and relative humidity (NSF). Dry-bulb temperature is simply the ambient air temperature and is unaffected by the moisture content of the air; dry-bulb temperature is measured by a standard thermometer (NSF). Wet-bulb temperature is the saturation temperature of air, and it is the lowest temperature at which water can evaporate into the air; this is measured using a thermometer with a moist cloth wrapped around the bulb (Padfield). Relative humidity is the amount of water vapor present in air, and is calculated as the ratio of partial pressure of water vapor to the equilibrium vapor pressure of water (Padfield). Figure 3 suggests strong correlations between air temperature moisture content in the air, measured as relative humidity. For example, at constant dry-bulb temperatures, the relative humidity increases as the wet-bulb temperature increases. Likewise, at constant wet-bulb temperatures, the relative humidity decreases as the dry-bulb temperature increases. In selecting the location of a chemical processing facility, especially for facilities where there is little control over the ambient temperature, it is important to understand that small changes in temperature can have exponential consequences on the moisture content/ relative humidity of the air; these temperature and humidity changes can affect both processing equipment and the materials being processed.
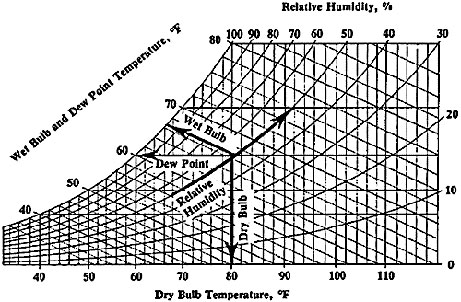
Weather Example
A variety of case studies have looked at weather effects on chemical processing. One such case explored the effects of temperature and humidity on phenol-formaldehyde resin bonding (Wang 253). Phenol-formaldehyde resin is a thermosetting adhesive that polymerizes and reacts with wood as part of the curing process in wood composite manufacturing. The strength of the resin bond is thought to be influenced by a variety of factors related to processing environment, including temperature and humidity. Figure 4 depicts the results from a study that compared the bond strength as a function of temperature, relative humidity, and bonding time (Wang 258-259).

As the results suggest, drastically different resin strength profiles are expected depending on relative humidity. Considering just the samples that were bonded at 110 ºC, the resins that were cured at 41% relative humidity overall cured stronger than their counterparts that were cured at the same time but at higher relative humidities. An interesting feature that is prevalent in the 110 ºC bonding samples is that processing conditions at higher relative humidities is not always indicative of a depreciated bond strength. As the graph suggests between the 75% and 90% relative humidity samples, when bonded for less than 10 minutes, the 90% relative humidity samples actually are stronger than the 75% samples. However, after about 10 minutes the trend exists such that samples cured at higher relative humidities overall bond more weakly.
There also appear to be striking differences between the 110 ºC and 120 ºC samples. In fact, it appears that less bonding time is required for the 120 ºC as is the time required to get comparable strengths for the 110 ºC samples. Also, at higher processing temperature of 120 ºC, it is evident that the samples at 75% relative humidity now can have comparable bond strengths as the samples at 41% relative humidity. Additionally, the samples at the 90% relative humidity also have somewhat higher binding strengths for the 120 ºC bonding temperatures compared to the 110 ºC bonding temperatures.
Thus, this study indicates the appreciable differences that can exist in the product quality based on humidity and temperature effects. Thus, depending on the desired product qualities (bond strength in this resin study), humidity and temperature are critical metrics in defining the process environment. This phenol-formaldehyde resin study is particularly useful in demonstrating the effects of ambient relative humidity on the mechanical strength of the product, and relative humidity is definitely a parameter that could fluctuate depending on the weather patterns of the processing environment. Additionally, 10 ºC (the difference between bonding at 110 ºC and 120 ºC) is well within the monthly and seasonal temperature fluctuations of different locations; whether or not the weather could be attributed to such processing differences at these high temperatures is a possibility.
The availability and price of raw materials for feed streams often play a large part in determining the plant location. For example, many ethylene plants are built in the Middle East near supplies of natural gas.
If building a plant near raw materials is not possible, often the next determining factor is ease of transportation. For most chemicals, proximity to major road, rail, waterway, or ports are desired. For high-value products such as pharmaceuticals, proximity to air ports can be used to prevent degradation of product during transport. Ease of transportation results in cheaper logistics cost for transport between both suppliers and buyers.
Proximity to utilities are important in chemical process. Water is ubiquitous in chemical plants and are often require in substantial amounts. Construction of plants near rivers and lakes are often desired to reduce the cost of process water. If drawing from local water is not possible, cooling towers will need to be used. Electrical power is required in all plants, often requiring plants to be built on available power grids.
Laws and regulations
Federal laws will be listed as it serves as a baseline for the entire country. State and local laws sometimes are stricter than the established federal laws resulting. Property costs, property taxes, corporate income taxes, and fines also vary between states. Therefore, further consultation of the state and local laws must also be done beyond the laws listed in this text to ensure adherence to all laws required for the location of the plant. Below are several hallmark federal laws which proper treatment and disposal of waste in the air, ground, and water (Towler and Sinnott, 2013).
The Clean Air Act
The Clean Air Act (CAA) was first passed in 1970 and was amended in 1990. The CAA empowers the EPA to set National Ambient Air Quality Standards (NAAQS)for the following seven contaminants:
- Ozone
- Carbon Monozide
- Lead
- Nitrogen Dioxide
- Sulfur Dioxide
- PM10: Partulate matter with mean diameter less than 10 μm.
- PM2.5: Partulate matter with mean diameter less than 2.5 μm.
In addition to these seven contaminatns, the CAA also empowered EPA to regulate an additional 189 hazardous air pollutants listed in the National Emission Standards for Hazardous Air Pollutants. Failure to meet NAAQS levels will result in the requirement of remediation steps to be taken to lower emissions before the plant is allowed to be operational.
The Clean Water Act
The Clean Water Act was first passed in 1972 and was amended in 1977 and 1987. It seeks to achieve clean water for swimming, boating, and protecting fish and water life. This act mandates that a permit from the EPA must be issued for discharge of pollutants into navigable waters.
The Safe Drinking Water Act
The Safe Drinking Water Act was passed in 1974. It empowers the EPA to set standards on the required purity of any water which could be used for drinking.
The Resource Conservation and Recovery Act
The Resource Conservation and Recovery Act was passed in 1976 to protect groundwater from contamination. This Act states that all waste producers are legally liable at any time from waste production to final disposal. Hazardous waste must be clearly labeled and tracked in transport and treated to levels specified by the EPA.
Waste Minimization and Management
Waste Minimization
Production of waste is arises naturally in any plant and require a noticeable amount of resources to take care of. Before even considering methods of managing ways, cost can significantly be reduced by efficient management by source reduction. Below is a five-step review often conducted to minimize waste production (Towler and Sinnott, 2013):
- 1. Identify waste: Identify what waste products are produced.
- 2. Economical Impact: Determine the size of the waste stream and the cost of treatment
- 3. Root causes: Determine the root cause of the waste streams.
- 4. Modifications: Analyze the effectiveness and cost of potential solutions.
- 5. Implement: Implement and optimize solutions of waste management.
Below are some source reduction strategies which can be employed (Towler and Sinnott, 2013):
- Purification of feeds: Impurities in feed streams can lead to side reactions and formation of waste. Either purchase of purer feeds or employment of purification techniques which do not generate more waste can be used. Purification of feeds will also lead to the reduction of purge and vent streams.
- Protect catalysis and adsorbents: Catalysts are sensitive to containment in the feed and be deactivated. One method of protecting the catalyst or adsorbent is by employing a guard bed of material to absorb or filter out contaminants.
- Eliminate use of extraneous materials: Limiting the diversity of solvents is beneficial. The mixing of different solvents can result in waste formation when solvents are degraded.
- Increase recovery from separations: Higher product recovery results in lower concentrations of products in the the waste streams and less waste formation.
- Improve fuel quality: Cleaner-burning fuel can have less harmful emissions.
- Recycle or sell side-products: Rather than processing side-products, attempt to identify companies which might use the waste as raw material for another process.
Keep in mind for all the strategies which can be employed to minimize waste production and therefore waste treatment, the overall cost must be considered. The savings from minimizing waste must be more than the additional cost implementing minimization.
Waste Management
There are many methods of waste treatment and safe disposal. The availability and efficiency of these methods depend heavily on location. Adherence to federal, state, and local laws may further restrict the availability, of some of these techniques. Common techniques include:
- Dilution and dispersion.
- Discharge into municipal sewer.
- Physical treatments: absorption, adsorption.
- Chemical treatment: precipitation, neutralization.
- Biological treatment: composting, anaerobic digestion.
- Incineration.
- Landfill at controlled sites.
- Sea dumping.
Economical definition of cost
Cost
All of the above criteria ultimately influence the capital and operating costs of a plant, and its expected lifespan.
Local wages, prices of chemical feedstock, shipping costs, and utilities all contribute to total operating costs.
Property prices, rental fees, taxes, and existing company property in the area contribute to recurring investment costs.
Site Layout: Design and Construction
Site Layout
The needs of a site for a chemical process vary considerably from process to process. In general, however, all chemical plants require the following (Towler and Sinnott, 2013):
- Shipping and receiving of products and raw materials
- Storage
- Utilities
- Offices and laboratories for management and quality control personnel
- Medical and fire services for emergency management
- Cafeterias, parking lots, and other amenities for employees.
These auxiliary buildings are often referred to as ancillary structures and they are placed within a chemical process to minimize transportation of goods and personnel, and to maximize safety. The following procedure is followed when determining the site layout of a chemical process (Mecklenburgh, 1985):
- 1. Major process equipment is placed in a logical order to minimize transportation of process streams. Extra emphasis is placed on the separation and treatment of hazardous materials as quickly as possible.
- 2. Utilities such as boilers and power plants are placed to minimize transportation of utility to its use within the process. Utilities are usually consolidated into one section of the chemical plant because they are usually generated together. For example, a boiler produces high pressure steam; half the steam is sent through a turbine to generate electricity and to expand the steam into low pressure steam.
- 3. Shipping and receiving are placed wherever there is a need to conform to preexisting infrastructure. For example, if the plant is located on a harbor, shipping and receiving for all barge shipments are located by the water. If the plant is built next to a railway, shipping by rail is located next to the tracks.
- 4. Storage tanks and warehouses are consolidated as much as possible. Storage of raw materials and products are stored between where they enter or exit the process and where they are shipped or received.
- 5. Ancillary buildings are placed to minimize time personnel spend traveling around the site.
- 6. Offices and labs are located as far away from hazardous processes as possible.
- 7. Walkways and roadways are added as needed to assist with construction and transportation during plant operation.
Site Construction
Site construction, along with process design, is an iterative process that follows a multi-step procedure (Mecklenburgh, 1985).
Stage One Layout
The "Proposal" or Stage One layout is the first step towards designing a site layout. The purpose of the Stage One layout is to assess the feasibility of the process according to the cost, hazard, risk, and environmental standards set by the interested parties.
The information included in a Stage One layout is the relative position of buildings and process equipment, and any other data that may come from a preliminary case study of a particular process. Additionally, preliminary estimates by manufacturers and contractors for process equipment and ancillary structures, as well as local building codes and regulations are used in generating the Stage One Layout.
Usually, different layouts for the same process may produce different costs. At this stage in development, many different layouts should be generated and the different layouts should be compared in a systematic way. It is usually very difficult to tell which layout is superior based purely on inspection. Once a Stage One design is finalized, the layout can move on to the next stage.
Stage Two Layout
Stage Two Layouts are generated based on the finalized Stage One design. Changes to the Stage One design are minimized. The purpose of the Stage Two Layout is to determine an accurate detailed cost of the entire process. Additionally, detailed hazard and environmental information is determined and submitted to all involved regulatory parties at this stage.
Final Stage Layout
After the detailed cost information, hazard, and environmental information are approved, the Final Design layout commences. At this stage, detailed drawings of all equipment, piping, and layouts are finalized. At the conclusion of the Final Stage layout, orders with contractors are placed and fabrication of process equipment begins, and the site land is purchased. Essentially, this is the "point of no return."
Construction
The first step in constructing the plant is remediation and preparation of the land for construction of a chemical plant. This can include clearing the land of trees and vegetation, removing other natural obstacles such as boulders and ditches, implementing a drainage system, landscaping, grading to remove difficult topography, and anything else that is necessary. Site selection attempts to minimize costs associated with this step, but there is invariably some form of preparation required for every site.
The second step is to construct all roadways, sidewalks, and fences required for both plant operation and plant construction. Costs associated with this step can range from 2 to 10 percent of the total capital investment for a chemical plant (Peters et al., 2002).
Process equipment and buildings are then constructed as soon as they are available. While construction schedules vary considerably from process to process, in some cases it is possible to perform the final construction steps once the process has already begun to operate, and the construction schedule is designed with this in mind (Mecklenburgh, 1985).
A new aspect of construction of process equipment is a modular approach, where process equipment is assembled as completely as possible by the manufacturer and shipped while assembled. The advantage to this approach is a more comprehensive testing of the equipment by the manufacturer and less installation time once the equipment has arrived on site (Towler and Sinnott, 2013).
References
Booth WM. Transactions of the American Institute of Chemical Engineers. Volume VI. New York: D Van Nostrond Co; 1913.
Mecklenburgh JC. Process plant layout. New York: Halsted Press; 1985.
National Science Foundation. "Hands-on Activity: Swamp Cooler." Teach Engineering. College of Engineering at University of Colorado Boulder and Integrated Teaching and Learning, 19 Feb. 2016. Web. 21 Feb. 2016. <https://www.teachengineering.org/view_activity.php?url=collection/cub_/activities/cub_housing/cub_housing_lesson01_activity2.xml>.
Padfield, Tim. "Glossary of the Microclimate Variables and Units Used in Conservation Physics." Conservation Physics. National Museum of Denmark, 14 June 2012. Web. 21 Feb. 2016. <http://www.conservationphysics.org/cpw/Storage/Fundamentals>.
Peters MS, Timmerhaus KD, West RE. Plant Design and Economics for Chemical Engineers. 5th ed. New York: McGraw-Hill; 2002.
Towler G, Sinnott R. General Site Considerations. In: Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.
Wang, Xiang-Ming, Bernard Riedl, Alfred Christiansen, and W. Geimer. "The Effects of Temperature and Humidity on Phenol-formaldehyde Resin Bonding." Wood Science and Technology 29.4 (1995): 253-66. Web.
"World Weather & Climate Information." Weather and Climate: Chicago, United States of America. World Weather and Climate Information, 2015. Web. 20 Feb. 2016. <https://weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine,Chicago,United-States-of-America>.