Desalination - Team G: Difference between revisions
No edit summary |
No edit summary |
||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
Team G: | Team G: Newport Desalination Plant | ||
Authors: KC Anderson, Neil Dalvie, Watson Fu, Helen Wu | Authors: KC Anderson, Neil Dalvie, Watson Fu, Helen Wu | ||
| Line 21: | Line 21: | ||
=Introduction= | =Introduction= | ||
As the human population increases, so does the need for new sources of freshwater. Global water use is currently at 9 trillion cubic meters per year and is expected to increase by about 60 billion cubic meters per year. In addition, droughts in the west coast of the United States have made the need for new sources of freshwater much more urgent in recent years. Growing urban populations in developed countries also have high requirements for water, and 39% of global population lives within 100 | As the human population increases, so does the need for new sources of freshwater. Global water use is currently at 9 trillion cubic meters per year and is expected to increase by about 60 billion cubic meters per year. In addition, droughts in the west coast of the United States have made the need for new sources of freshwater much more urgent in recent years. Growing urban populations in developed countries also have high requirements for water, and 39% of global population lives within 100 kilometers of an ocean coast.<sup>1</sup> This means that a large percentage of people do not have access to fresh water sources. Because of these concerns, a large market exists for desalinated water. As of 2013, desalination plants produced 78.4 million cubic meters of water per day and this number is expected to increase.<sup>2</sup> | ||
There are two main categories of methods of desalination used in industry. The first category is thermal-based separation. Multi-Stage Flash Distillation (MSF) has been widely utilized and involves heating and pressurizing impure water to separate water vapor. MSF is the most popular thermal separation method because of the high purity that can be obtained.3 The second category is membrane-based separation. Reverse Osmosis (RO) is becoming the preferred method in industry. RO uses a pressure gradient to drive water through a membrane. Compared to most other methods, RO has low energy requirements and higher yield.4 | There are two main categories of methods of desalination used in industry. The first category is thermal-based separation. Multi-Stage Flash Distillation (MSF) has been widely utilized and involves heating and pressurizing impure water to separate water vapor. MSF is the most popular thermal separation method because of the high purity that can be obtained.<sup>3</sup> The second category is membrane-based separation. Reverse Osmosis (RO) is becoming the preferred method in industry. RO uses a pressure gradient to drive water through a membrane. Compared to most other methods, RO has low energy requirements and higher yield.<sup>4</sup> | ||
The purpose of this report is to examine the potential implementation of a MSF desalination plant and evaluate the economic feasibility of the design. The remainder of the report outlines the process design, economics of the design, and important recommendations to further optimize the design and increase economic feasibility. | The purpose of this report is to examine the potential implementation of a MSF desalination plant and evaluate the economic feasibility of the design. The remainder of the report outlines the process design, economics of the design, and important recommendations to further optimize the design and increase economic feasibility. | ||
| Line 31: | Line 31: | ||
===Location=== | ===Location=== | ||
This desalination plant will be located in Newport, Oregon to provide water to the mid-coastal region of Oregon. Oregon has recently suffered a major drought, and 23 out of 36 counties implemented agricultural water regulation and applied for federal assistance.5 As 2016 arrives, Oregon has ended its state drought emergency, but many new water regulations and conservation efforts appear to be permanent going forward. Oregon also offers an abundance of and commitment to clean energy not seen in other states. In California, desalination in reaction to extreme drought has forced plants to rely on destructive fossil fuel energy sources, thereby offsetting the environmental effect of desalination. A desalination process in Oregon would be posed to take advantage of a high and growing grid of renewable power. So far, 73% of Oregon’s electricity generation is renewable.6 The town of Newport boasts proximity to free coastline, and separation from major wildlife and forest reserves. In 2013, Oregon State University selected Newport as the location for its Pacific Marine Energy Center, a large scale trial of renewable wave energy.7 Independent of the efficacy of wave energy, this project indicates the overall availability and commitment to renewable energy in Newport. Finally, while it is slightly farther from the drought stricken southern Oregon counties that are most affected by the California shortage, Newport lies in proximity to the agriculture-rich and highly water-dependent agriculture in the Willamette valley.8 | This desalination plant will be located in Newport, Oregon to provide water to the mid-coastal region of Oregon. Oregon has recently suffered a major drought, and 23 out of 36 counties implemented agricultural water regulation and applied for federal assistance.<sup>5</sup> As 2016 arrives, Oregon has ended its state drought emergency, but many new water regulations and conservation efforts appear to be permanent going forward. Oregon also offers an abundance of and commitment to clean energy not seen in other states. In California, desalination in reaction to extreme drought has forced plants to rely on destructive fossil fuel energy sources, thereby offsetting the environmental effect of desalination. A desalination process in Oregon would be posed to take advantage of a high and growing grid of renewable power. So far, 73% of Oregon’s electricity generation is renewable.<sup>6</sup> The town of Newport boasts proximity to free coastline, and separation from major wildlife and forest reserves. In 2013, Oregon State University selected Newport as the location for its Pacific Marine Energy Center, a large scale trial of renewable wave energy.<sup>7</sup> Independent of the efficacy of wave energy, this project indicates the overall availability and commitment to renewable energy in Newport. Finally, while it is slightly farther from the drought stricken southern Oregon counties that are most affected by the California shortage, Newport lies in proximity to the agriculture-rich and highly water-dependent agriculture in the Willamette valley.<sup>8</sup> | ||
===Process Requirements=== | ===Process Requirements=== | ||
This plant will produce 15,500 cubic meters of desalinated water per day, aiming to offset on the order of ~10% of predicted increase in out-of-stream water demand in the mid-coastal region in coming years. The feed for this process is only seawater, sourced from the Newport coastal water with a salinity of 32 PSU (g/kg of seawater).9 The process will produce 99.2% desalinated, potable water with a maximum chloride concentration of 250 mg/L and total dissolved solids of 500 mg/L. As waste, the process will release diluted, cooled, brine from a surge tank. Composition of the feed seawater can be found in Appendix E. | This plant will produce 15,500 cubic meters of desalinated water per day, aiming to offset on the order of ~10% of predicted increase in out-of-stream water demand in the mid-coastal region in coming years. The feed for this process is only seawater, sourced from the Newport coastal water with a salinity of 32 PSU (g/kg of seawater).<sup>9</sup> The process will produce 99.2% desalinated, potable water with a maximum chloride concentration of 250 mg/L and total dissolved solids of 500 mg/L. As waste, the process will release diluted, cooled, brine from a surge tank. Composition of the feed seawater can be found in Appendix E. | ||
=Technical Approach= | =Technical Approach= | ||
We decided to use Multi-Stage Flash Distillation (MSF) for the desalination process in our plant. Principally, MSF allows us to achieve the purity required for Oregon regulations. Oregon water regulations include an upper limit of salt concentration at 250 mg/L for potable water.10 Because of this, MSF provides a more reliable high purity product than does reverse osmosis, the main alternative. In addition, thermal methods like MSF achieve the desired purity with less dependence on input conditions. While we expect seawater concentrations to remain largely constant, a robust process is desirable. While membranes require significant pretreatment of feeds, thermal methods can process raw seawater and do not run the risk of microbial contamination.4 Despite this advantage, MSF typically sees considerably lower yield, and higher thermal energy costs than reverse osmosis.11 This decision was made after considering a number of options, described in this section. Design alternatives are based on a simple separation block diagram, shown in Appendix A. | We decided to use Multi-Stage Flash Distillation (MSF) for the desalination process in our plant. Principally, MSF allows us to achieve the purity required for Oregon regulations. Oregon water regulations include an upper limit of salt concentration at 250 mg/L for potable water.<sup>10</sup> Because of this, MSF provides a more reliable high purity product than does reverse osmosis, the main alternative. In addition, thermal methods like MSF achieve the desired purity with less dependence on input conditions. While we expect seawater concentrations to remain largely constant, a robust process is desirable. While membranes require significant pretreatment of feeds, thermal methods can process raw seawater and do not run the risk of microbial contamination.<sup>4</sup> Despite this advantage, MSF typically sees considerably lower yield, and higher thermal energy costs than reverse osmosis.<sup>11</sup> This decision was made after considering a number of options, described in this section. Design alternatives are based on a simple separation block diagram, shown in Appendix A. | ||
==Process Alternatives== | ==Process Alternatives== | ||
| Line 46: | Line 46: | ||
===Pressure Control Design Options=== | ===Pressure Control Design Options=== | ||
An important aspect of most desalination processes is establishing a pressure gradient. In membrane technologies, the pressure gradient is a driving force for separation against a concentration gradient. Forward osmosis holds a major advantage in this section of the process, as little to no gauge pressure is required to drive osmosis.12 In comparison, reverse osmosis requires high levels of pressure to achieve separation.13 The magnitude of the pressures increases capital costs and utilities costs tremendously, which is a significant disadvantage. For thermal separation technology, low pressure works in accordance with the thermal changes to remove steam from the concentrated brine, as the water vapor saturation temperature changes with changing pressure. The two main methods of vacuum creation are seawater eductors and vacuum pumps. An eductor is convenient when high energy flows are accessible within the process. In the absence of extra flows, we decided to utilize a simple vacuum pump. While energy intensive, this pump achieves low pressures easily. | An important aspect of most desalination processes is establishing a pressure gradient. In membrane technologies, the pressure gradient is a driving force for separation against a concentration gradient. Forward osmosis holds a major advantage in this section of the process, as little to no gauge pressure is required to drive osmosis.<sup>12</sup> In comparison, reverse osmosis requires high levels of pressure to achieve separation.<sup>13</sup> The magnitude of the pressures increases capital costs and utilities costs tremendously, which is a significant disadvantage. For thermal separation technology, low pressure works in accordance with the thermal changes to remove steam from the concentrated brine, as the water vapor saturation temperature changes with changing pressure. The two main methods of vacuum creation are seawater eductors and vacuum pumps. An eductor is convenient when high energy flows are accessible within the process. In the absence of extra flows, we decided to utilize a simple vacuum pump. While energy intensive, this pump achieves low pressures easily. | ||
===Pretreatment Design Options=== | ===Pretreatment Design Options=== | ||
Membrane technologies, including forward and reverse osmosis, are limited by the size and selectivity of the membrane. This presents an issue, as Oregon mandates strict upper limits on organic contaminants.14 One solution to this issue is to source water from either several hundred meter depth or from beach wells, where water has already passed through sediment.15 In addition to feed requirements, reverse osmosis methods require several pretreatment steps to avoid severe membrane fouling.13 Forward osmosis processes require the addition of a draw solution on the permeate side of the membrane to create an osmotic pressure driving force.12 Thermal desalination relies on the heating of seawater to obtain a pure distillate. In early design stages, we considered the implementation of a refrigeration loop. Unfortunately, the purchase of refrigerants are prohibitively expensive,16 and a refrigeration loop is beneficial when heat needs to be transferred from one area of the process to another. With the implementation of a vacuum pump, there is nothing in the process that needs to be cooled. For this reason and cost, we decided to heat our process stream using a condensing steam heat exchanger. | Membrane technologies, including forward and reverse osmosis, are limited by the size and selectivity of the membrane. This presents an issue, as Oregon mandates strict upper limits on organic contaminants.<sup>14</sup> One solution to this issue is to source water from either several hundred meter depth or from beach wells, where water has already passed through sediment.<sup>15</sup> In addition to feed requirements, reverse osmosis methods require several pretreatment steps to avoid severe membrane fouling.<sup>13</sup> Forward osmosis processes require the addition of a draw solution on the permeate side of the membrane to create an osmotic pressure driving force.<sup>12</sup> Thermal desalination relies on the heating of seawater to obtain a pure distillate. In early design stages, we considered the implementation of a refrigeration loop. Unfortunately, the purchase of refrigerants are prohibitively expensive,<sup>16</sup> and a refrigeration loop is beneficial when heat needs to be transferred from one area of the process to another. With the implementation of a vacuum pump, there is nothing in the process that needs to be cooled. For this reason and cost, we decided to heat our process stream using a condensing steam heat exchanger. | ||
===Separation Design Options=== | ===Separation Design Options=== | ||
One of the main separation methods for desalination is membrane separation. Forward osmosis relies on a membrane to allow transfer of water under purely osmotic forces. However, continuous flow is difficult to arrange spatially since the concentrated draw solution must be recycled back through the system. Very little literature exists on practical uses of forward osmosis membranes for desalination, so we have chosen to avoid this option. Reverse osmosis uses hydraulic pressure to force osmosis, rather than a draw solution and concentration gradient. Reverse osmosis can generally achieve only 98% salt removal, requiring multiple passes.12 A vast majority of MSF processes are centered around a series of flash chambers with descending pressure and temperature. Vaporized water is collected in a tray as the pure distillate, with increasingly concentrated brine flowing into the next flash chamber. In order to maintain the pressure gradient needed, a vacuum pump is used. By aligning the flash chambers into one unit, only one pump would be needed to create the pressure gradient, reducing both capital and operating costs.17 Therefore, we have decided to move forward with MSF with the use of flash chambers connected into one unit for our separation. | One of the main separation methods for desalination is membrane separation. Forward osmosis relies on a membrane to allow transfer of water under purely osmotic forces. However, continuous flow is difficult to arrange spatially since the concentrated draw solution must be recycled back through the system. Very little literature exists on practical uses of forward osmosis membranes for desalination, so we have chosen to avoid this option. Reverse osmosis uses hydraulic pressure to force osmosis, rather than a draw solution and concentration gradient. Reverse osmosis can generally achieve only 98% salt removal, requiring multiple passes.<sup>12</sup> A vast majority of MSF processes are centered around a series of flash chambers with descending pressure and temperature. Vaporized water is collected in a tray as the pure distillate, with increasingly concentrated brine flowing into the next flash chamber. In order to maintain the pressure gradient needed, a vacuum pump is used. By aligning the flash chambers into one unit, only one pump would be needed to create the pressure gradient, reducing both capital and operating costs.<sup>17</sup> Therefore, we have decided to move forward with MSF with the use of flash chambers connected into one unit for our separation. | ||
===Waste Treatment Design Options=== | ===Waste Treatment Design Options=== | ||
Reverse osmosis typically requires additional steps to return the water product to an acceptable pH after the initial acidification before release, in addition to dilution.13 Forward osmosis technology requires separation of pure water from the draw solution through heating. This adds significantly to the otherwise minimal energy requirement of a forward osmosis process.12 One technology that could improve waste treatment for an MSF process is adding a brine recycle. Two methods of concentrated brine recycle are prevalent. In one method, a portion of concentrated brine is recycled into the seawater feed, with the rest of the brine sent to dilution and waste.18 Alternatively, concentrated bring can be rerouted to a surge tank. This tank is controlled to maintain a concentration acceptably diluted for waste, serving as the seawater feed and the waste “purge”, with the two having the same composition.17 Traditionally in chemical processes, recycle systems require more energy to carry out the process. Because desalination is itself a separation, recycle may be advantageous because of the retained heat energy. In the second recycle method, the surge tank serves not only to cool the diluted waste to an acceptable release temperature, but also to preheat the process feed. In this setup, where no heat is rejected into the waste, thermal efficiency may actually increase, decreasing utility costs.18 For these reasons, we have decided to implement a surge tank recycle stream. | Reverse osmosis typically requires additional steps to return the water product to an acceptable pH after the initial acidification before release, in addition to dilution.<sup>13</sup> Forward osmosis technology requires separation of pure water from the draw solution through heating. This adds significantly to the otherwise minimal energy requirement of a forward osmosis process.<sup>12</sup> One technology that could improve waste treatment for an MSF process is adding a brine recycle. Two methods of concentrated brine recycle are prevalent. In one method, a portion of concentrated brine is recycled into the seawater feed, with the rest of the brine sent to dilution and waste.<sup>18</sup> Alternatively, concentrated bring can be rerouted to a surge tank. This tank is controlled to maintain a concentration acceptably diluted for waste, serving as the seawater feed and the waste “purge”, with the two having the same composition.<sup>17</sup> Traditionally in chemical processes, recycle systems require more energy to carry out the process. Because desalination is itself a separation, recycle may be advantageous because of the retained heat energy. In the second recycle method, the surge tank serves not only to cool the diluted waste to an acceptable release temperature, but also to preheat the process feed. In this setup, where no heat is rejected into the waste, thermal efficiency may actually increase, decreasing utility costs.<sup>18</sup> For these reasons, we have decided to implement a surge tank recycle stream. | ||
=Results= | =Results= | ||
| Line 65: | Line 65: | ||
Once the overall design equipment and strategy was selected, mass and energy balances were calculated and optimized for yield and cost. To determine these values, temperatures, and flow rates, we made a number of assumptions and set points in our process. The feed and waste concentrations were held constants, at the composition of Oregon sea water, and the maximum allowable waste concentration. The flash inlet was held at 1 atm and 98°C, in order to maximize energy carried by the stream without premature boiling. The distillate flow rate was held constant in line with our initial problem statement and project goals. Finally, phase data was obtained from Aspen+. While true seawater will contain other contaminants, these have small effects on thermodynamic properties. Pretreatment and material selection will take additional contaminants into consideration, but they are neglected in mass and energy calculations. | Once the overall design equipment and strategy was selected, mass and energy balances were calculated and optimized for yield and cost. To determine these values, temperatures, and flow rates, we made a number of assumptions and set points in our process. The feed and waste concentrations were held constants, at the composition of Oregon sea water, and the maximum allowable waste concentration. The flash inlet was held at 1 atm and 98°C, in order to maximize energy carried by the stream without premature boiling. The distillate flow rate was held constant in line with our initial problem statement and project goals. Finally, phase data was obtained from Aspen+. While true seawater will contain other contaminants, these have small effects on thermodynamic properties. Pretreatment and material selection will take additional contaminants into consideration, but they are neglected in mass and energy calculations. | ||
[[File:Watson_PFD_appendixB.JPG|frame|center|border|<div align=center> ASPEN+ simulation <div>]] | |||
<div align=left> | |||
===Pressure Considerations and Yield=== | ===Pressure Considerations and Yield=== | ||
| Line 70: | Line 73: | ||
Aspen+ phase data revealed that because the energy used to vaporize the water is carried in the inlet stream, the amount of water flashed depends almost completely on the pressure in the last flash stage, or the lowest pressure in the process. Because of this, the mass balances over the entire process are largely dependent on the equilibrium conditions in the last drum. Therefore, for overall balances, we treated the connected series of flash drums as one unit. This assumption is based on the adiabatic nature of the drums, and the assumption that the brine reaches phase equilibrium before leaving the unit. This yields a simplified block diagram for the purpose of calculating overall mass balances, as shown in Appendix A. Figure 1a shows conditions at a range of vacuum pressures. As pressure is decreased, the yield of vaporization increases, which corresponds to an increase in the outlet concentration of NaCl for recycle. Temperature decreases with pressure to maintain vapor-liquid saturation conditions. The temperature profile is critical in designing the multistage flash unit, as higher temperatures through the pressure gradient will release hot distillate that can be captured in preheating. | Aspen+ phase data revealed that because the energy used to vaporize the water is carried in the inlet stream, the amount of water flashed depends almost completely on the pressure in the last flash stage, or the lowest pressure in the process. Because of this, the mass balances over the entire process are largely dependent on the equilibrium conditions in the last drum. Therefore, for overall balances, we treated the connected series of flash drums as one unit. This assumption is based on the adiabatic nature of the drums, and the assumption that the brine reaches phase equilibrium before leaving the unit. This yields a simplified block diagram for the purpose of calculating overall mass balances, as shown in Appendix A. Figure 1a shows conditions at a range of vacuum pressures. As pressure is decreased, the yield of vaporization increases, which corresponds to an increase in the outlet concentration of NaCl for recycle. Temperature decreases with pressure to maintain vapor-liquid saturation conditions. The temperature profile is critical in designing the multistage flash unit, as higher temperatures through the pressure gradient will release hot distillate that can be captured in preheating. | ||
[[File:Watson f1.JPG|frame|center|border|<div align=center> Figure 1. a) Flash conditions with respect to pressure in the last drum. b) Process metrics with respect to pressure in the last drum. <div>]] | |||
<div align=left> | |||
At first inspection, it appears advantageous to operate at the lowest possible pressure to obtain the highest vaporization yield. However, dilution for waste proved to be a more significant factor in overall process yield than the yield over the flash drum unit. Operating at the lowest possible pressure maximizes vapor yield, but creates a more concentrated recycle stream. This higher concentration requires more process feed to dilute to waste conditions, lowering the overall process yield. For this reason, it is desirable to produce a recycle stream as close to waste concentration as possible, minimizing the amount of process feed needed to dilute to waste conditions. Figure 1b shows the effect of flash pressure on overall process metrics. It becomes clear that the overall yield increases with pressure as an asymptote. Above a certain pressure, the recycle stream becomes too dilute to create a waste concentration of 40 g/kg, creating a negative feed requirement for this calculation. Because we would like to release waste of 40 g/kg, we focus on the feasible solutions below 0.4 atm. Figure 1b also shows the small effect on heating requirements as the pressure is changed. Because the amount of water vaporized is held constant, this energy is largely representative of the energy needed to vaporize that amount of water. Figure 1a shows that at higher operating pressures and lower vaporization yields, the brine recycle will remain hot. Therefore, despite increased recycle rates, the higher temperature keeps the energy requirement nearly constant. With these considerations, we will operate at a flash pressure that limits the vaporization yield, keeping the recycle stream near waste concentrations. When operating at a pressure of 0.3 atm, an overall yield of approximately 62% can be achieved. This higher pressure will also provide energy savings in vacuum creation. | At first inspection, it appears advantageous to operate at the lowest possible pressure to obtain the highest vaporization yield. However, dilution for waste proved to be a more significant factor in overall process yield than the yield over the flash drum unit. Operating at the lowest possible pressure maximizes vapor yield, but creates a more concentrated recycle stream. This higher concentration requires more process feed to dilute to waste conditions, lowering the overall process yield. For this reason, it is desirable to produce a recycle stream as close to waste concentration as possible, minimizing the amount of process feed needed to dilute to waste conditions. Figure 1b shows the effect of flash pressure on overall process metrics. It becomes clear that the overall yield increases with pressure as an asymptote. Above a certain pressure, the recycle stream becomes too dilute to create a waste concentration of 40 g/kg, creating a negative feed requirement for this calculation. Because we would like to release waste of 40 g/kg, we focus on the feasible solutions below 0.4 atm. Figure 1b also shows the small effect on heating requirements as the pressure is changed. Because the amount of water vaporized is held constant, this energy is largely representative of the energy needed to vaporize that amount of water. Figure 1a shows that at higher operating pressures and lower vaporization yields, the brine recycle will remain hot. Therefore, despite increased recycle rates, the higher temperature keeps the energy requirement nearly constant. With these considerations, we will operate at a flash pressure that limits the vaporization yield, keeping the recycle stream near waste concentrations. When operating at a pressure of 0.3 atm, an overall yield of approximately 62% can be achieved. This higher pressure will also provide energy savings in vacuum creation. | ||
| Line 76: | Line 80: | ||
===Flash Stage Optimization and Sizing=== | ===Flash Stage Optimization and Sizing=== | ||
The flash unit, where all flash stages occur, and makes up the bulk of the process. It consists of 9 vertical flash drums connected in series, each with a condenser in the upper portion. The drums are held at low pressure, allowing the volume to fill with saturated water vapor. This vapor condenses on heat exchange pipes in the top of the drum, and condenses, falling onto a collection tray. Once overall mass balances were calculated, detailed mass and energy balances on the major flash unit were analyzed. First, the equilibrium in each stage was characterized. Connected equilibrium stages exhibit linearly decreasing temperature.19 Optimization of mass balances called for a pressure of 0.3 atm in the last drum to achieve the highest yield. This produces the following equilibrium conditions across all 9 drums (the number of drums eventually selected). | The flash unit, where all flash stages occur, and makes up the bulk of the process. It consists of 9 vertical flash drums connected in series, each with a condenser in the upper portion. The drums are held at low pressure, allowing the volume to fill with saturated water vapor. This vapor condenses on heat exchange pipes in the top of the drum, and condenses, falling onto a collection tray. Once overall mass balances were calculated, detailed mass and energy balances on the major flash unit were analyzed. First, the equilibrium in each stage was characterized. Connected equilibrium stages exhibit linearly decreasing temperature.<sup>19</sup> Optimization of mass balances called for a pressure of 0.3 atm in the last drum to achieve the highest yield. This produces the following equilibrium conditions across all 9 drums (the number of drums eventually selected). | ||
[[File:Watson f2.JPG|frame|center|border|<div align=center> Figure 2. a) Final equilibrium temperature and pressure as a function of the number of flash drums. b) Cost of increasing number of drums. <div>]] | |||
<div align=left> | |||
Interestingly, this equilibrium profile creates nearly constant vapor flow rates in each drum. These flow rates, along with the density of saturated vapor in each drum, were used to calculate the minimum chamber diameter to allow enough vapor-liquid interface. The minimum diameter for the lowest pressure drum was approximately 6 m, and the minimum for preceding drums varied minimally. For manufacturing simplicity, we have decided to build all 9 drums using a diameter and height of 6 m. As explained previously, drums will be constructed of stainless steel to prevent corrosion. Because each drum will be operated in vacuum conditions, the pressure on drum walls will never exceed 1 atm. We therefore calculated the thickness of material based on structural feasibility, requiring a thickness of 3 cm. Pricing of the drums was approximated using the required weight of stainless steel. In addition to drum material, the cost of each condensing unit was approximated using the required heat exchange area to condense the distillate. This calculation was performed under the assumption that the surge tank outlet (See Appendix L) will be used as the cooling stream on the tube side. Approximate cost of each condensing system was derived heuristically from the heat transfer area. All of these equilibrium and costing calculations were repeated for a growing number of stages. Figure 2b indicates the total cost of the flash unit for different numbers of stages. Using this, we selected 9 tanks, within the region of diminishing returns. Once this number was selected, we began specific design of each condensing region of the drums. Notably, the last three drums require excessive heat transfer area that exceeds 5000 square meters. The last drum, which requires nearly 19000 square meters, can be remedied by using the process seawater feed to cool, which is at a significantly lower temperature than the surge tank. Unfortunately, the process feed does not have the heat capacity to cool the 7th and 8th drums. For this initial design and economic analysis, these drums retain an unrealistic heat transfer area. In implementation, additional cooling water would be required. The cost of demisters and trays were also considered. The approximate price for a 6-meter diameter demister is $500. Both the demister and condensing tray price were considered negligible compared to the overall price of the flash chambers. | Interestingly, this equilibrium profile creates nearly constant vapor flow rates in each drum. These flow rates, along with the density of saturated vapor in each drum, were used to calculate the minimum chamber diameter to allow enough vapor-liquid interface. The minimum diameter for the lowest pressure drum was approximately 6 m, and the minimum for preceding drums varied minimally. For manufacturing simplicity, we have decided to build all 9 drums using a diameter and height of 6 m. As explained previously, drums will be constructed of stainless steel to prevent corrosion. Because each drum will be operated in vacuum conditions, the pressure on drum walls will never exceed 1 atm. We therefore calculated the thickness of material based on structural feasibility, requiring a thickness of 3 cm. Pricing of the drums was approximated using the required weight of stainless steel. In addition to drum material, the cost of each condensing unit was approximated using the required heat exchange area to condense the distillate. This calculation was performed under the assumption that the surge tank outlet (See Appendix L) will be used as the cooling stream on the tube side. Approximate cost of each condensing system was derived heuristically from the heat transfer area. All of these equilibrium and costing calculations were repeated for a growing number of stages. Figure 2b indicates the total cost of the flash unit for different numbers of stages. Using this, we selected 9 tanks, within the region of diminishing returns. Once this number was selected, we began specific design of each condensing region of the drums. Notably, the last three drums require excessive heat transfer area that exceeds 5000 square meters. The last drum, which requires nearly 19000 square meters, can be remedied by using the process seawater feed to cool, which is at a significantly lower temperature than the surge tank. Unfortunately, the process feed does not have the heat capacity to cool the 7th and 8th drums. For this initial design and economic analysis, these drums retain an unrealistic heat transfer area. In implementation, additional cooling water would be required. The cost of demisters and trays were also considered. The approximate price for a 6-meter diameter demister is $500. Both the demister and condensing tray price were considered negligible compared to the overall price of the flash chambers. | ||
| Line 98: | Line 103: | ||
===Pumps=== | ===Pumps=== | ||
Based on sizing estimations given in | Based on sizing estimations given in Towler<sup>20</sup>, Ch. 7, P-101 will be $323,151, P-102 will be $12,446, and P-103 will be $311,369. The utilities can be calculated using the brake hp of the pumps. P-101 has a brake hp of 166,870 kW, P-102 has a brake hp of 4.1 kW, and P-103 has a brake hp of 457.37 kW. The utility cost for P-101 is large because of the pressure drop in the heater, E-101. Detailed design of pumps can be found in Appendices N-P. | ||
==Safety, Control, and Environmental Considerations== | ==Safety, Control, and Environmental Considerations== | ||
| Line 108: | Line 113: | ||
===Environmental Considerations=== | ===Environmental Considerations=== | ||
Our process does not include a significant post treatment, and produces de-ionized water because our plant must meet demands for both agriculture and human consumption. Because Oregon has recently left drought conditions, we believe that local treatment centers currently have additional capacity available. Oregon has strict regulations on the salinity of wastewater for marine health. Our process reaches but does not exceed the maximum salt concentration of 40g/L in wastewater. We plan to utilize clean energy for our process. Oregon offers an abundance of and commitment to clean energy not seen in other states. In California, desalination in reaction to extreme drought has forced plants to rely on destructive fossil fuel energy sources, thereby offsetting the environmental effect of desalination. A desalination process in Oregon would be posed to take advantage of a high and growing grid of renewable power. So far, 73% of Oregon’s electricity generation is renewable.6 | Our process does not include a significant post treatment, and produces de-ionized water because our plant must meet demands for both agriculture and human consumption. Because Oregon has recently left drought conditions, we believe that local treatment centers currently have additional capacity available. Oregon has strict regulations on the salinity of wastewater for marine health. Our process reaches but does not exceed the maximum salt concentration of 40g/L in wastewater. We plan to utilize clean energy for our process. Oregon offers an abundance of and commitment to clean energy not seen in other states. In California, desalination in reaction to extreme drought has forced plants to rely on destructive fossil fuel energy sources, thereby offsetting the environmental effect of desalination. A desalination process in Oregon would be posed to take advantage of a high and growing grid of renewable power. So far, 73% of Oregon’s electricity generation is renewable.<sup>6</sup> | ||
===Scaling and Corrosion=== | ===Scaling and Corrosion=== | ||
In the brine recycle stream, salt levels climb as high as 41 g/kg. While not extreme conditions, this salinity along with additional contaminants brings attention to the prevention of corrosion and control of scaling. We have decided to build our desalination plant with 316 stainless steel because of its excellent resistance to general and localized corrosion.21 Although 316 stainless steel is three times as expensive as carbon steel it will maintain efficient operation with less failures due to corrosion damage.20 We have decided to add an antiscalant to our process instead of acid addition.22 Acidification, although effective in preventing the precipitation of calcium carbonate, is relatively ineffective in preventing other types of scale and also less cost effective.23 Based on performance studies, we have decided to use a polyphosphate acid inhibitor as our antiscalant, at a dosing rate of 1.5ppm due to the relatively low maximum operating temperature of 98°C.22 | In the brine recycle stream, salt levels climb as high as 41 g/kg. While not extreme conditions, this salinity along with additional contaminants brings attention to the prevention of corrosion and control of scaling. We have decided to build our desalination plant with 316 stainless steel because of its excellent resistance to general and localized corrosion.<sup>21</sup> Although 316 stainless steel is three times as expensive as carbon steel it will maintain efficient operation with less failures due to corrosion damage.20 We have decided to add an antiscalant to our process instead of acid addition.<sup>22</sup> Acidification, although effective in preventing the precipitation of calcium carbonate, is relatively ineffective in preventing other types of scale and also less cost effective.<sup>23</sup> Based on performance studies, we have decided to use a polyphosphate acid inhibitor as our antiscalant, at a dosing rate of 1.5ppm due to the relatively low maximum operating temperature of 98°C.22 | ||
==Economic Evaluation and Sensitivity Analysis== | ==Economic Evaluation and Sensitivity Analysis== | ||
The ISBL capital costs were estimated to be 14.5 MM$, while OSBL costs were estimated to be 40% of ISBL costs. Individual equipment costs can be found in Appendix D. Since the plant is located on the West Coast, a location factor of 1.07 was applied. The variable cost of production for the plant has three main sources: raw materials, consumables, and utilities. The main raw materials costs are from the antiscalant, since we will not have to pay for the seawater feed; the antiscalant will cost $542,000/year for the flow of 100,000 | The ISBL capital costs were estimated to be 14.5 MM$, while OSBL costs were estimated to be 40% of ISBL costs. Individual equipment costs can be found in Appendix D. Since the plant is located on the West Coast, a location factor of 1.07 was applied. The variable cost of production for the plant has three main sources: raw materials, consumables, and utilities. The main raw materials costs are from the antiscalant, since we will not have to pay for the seawater feed; the antiscalant will cost $542,000/year for the flow of 100,000 m<sup>3</sup> of seawater per day. Utility costs mainly consisted of electricity for the pumps and steam for the heat exchanger. Detailed equipment and utility costs are shown in Appendix D. The total fixed capital cost was calculated to be 129.7 MM$. Major assumptions include having three shifts of five operators earning $50,000 salaries, maintenance of the plant at 5% of ISBL costs, and direct labor overhead being 25% of operator costs. | ||
Profitability of the plant was assessed by calculating the net present value (NPV) and internal rate of return (IRR). The price of purified water was assumed to be $3.00 per 1000 gallons from average water costs in Newport, Oregon and assuming we will have to sell our water at a lower price to treatment facilities before being sold for public use.24 Assuming a 5-year MACRS depreciation schedule, this plant is not profitable by a large margin, losing up to 7 billion | Profitability of the plant was assessed by calculating the net present value (NPV) and internal rate of return (IRR). The price of purified water was assumed to be $3.00 per 1000 gallons from average water costs in Newport, Oregon and assuming we will have to sell our water at a lower price to treatment facilities before being sold for public use.<sup>24</sup> Assuming a 5-year MACRS depreciation schedule, this plant is not profitable by a large margin, losing up to 7 billion dollar over a 20 year window. Main sources of the imbalance are high utility costs ($150 MM for high pressure steam). If profit is the goal of this process, we would not recommend pursuing this project. However, if there a pressing need and investment for purified water, and a potential source of local energy from renewable sources, this process may be a feasible project, though expensive. The full economic analysis can be found in Appendix J. A sensitivity analysis revealed that lowering high energy requirements is imperative to drive down prohibitive costs. In addition, subsidies would be necessary to execute this process. Sensitivity to several process parameters is represented in Figure 3. | ||
[[File:Watson f3.JPG|frame|center|border|<div align=center> Figure 3. Sensitivity analysis based on change in the 20 year projection. <div>]] | |||
<div align=left> | |||
=Conclusion= | =Conclusion= | ||
| Line 129: | Line 135: | ||
=Appendices= | =Appendices= | ||
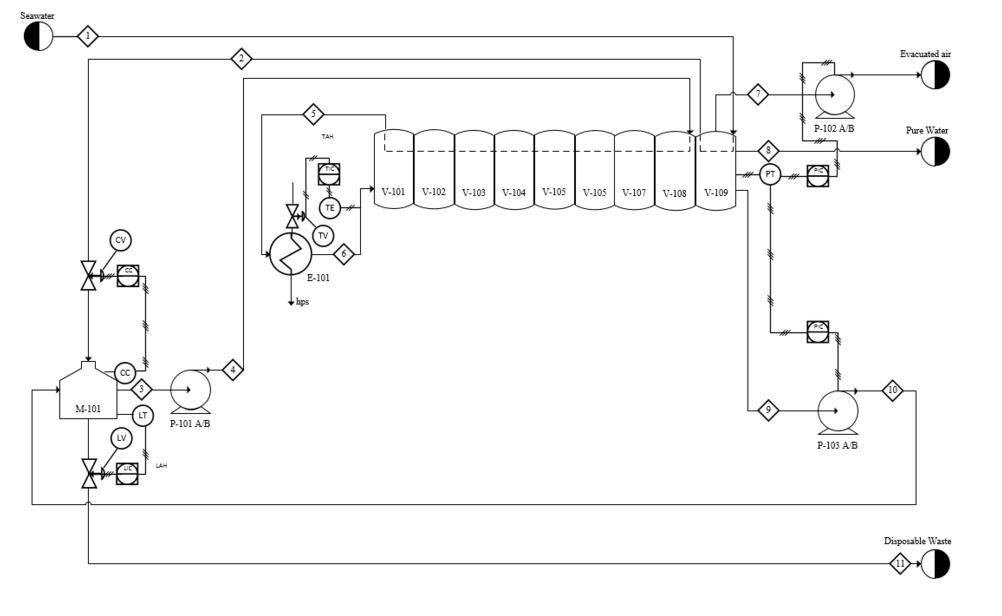
==Appendix A== | ==Appendix A: Block Flow Diagram== | ||
[[File:Watson appendixA.JPG|frame|center|border|<div align=center> <div>]] | |||
<div align=left> | |||
==Appendix B: Process flow diagram== | |||
[[File:Watson_PFD_appendixB.JPG|frame|center|border|<div align=center> <div>]] | |||
<div align=left> | |||
==Appendix C: Aspen Model=== | |||
[[File:Watson_aspen.JPG|frame|center|border|<div align=center> <div>]] | |||
<div align=left> | |||
==Appendix D: Equipment and Utility Costs=== | |||
[[File:Watson_utility_appD.JPG|frame|center|border|<div align=center> <div>]] | |||
<div align=left> | |||
[[File:Watson equip appD.JPG|frame|center|border|<div align=center> <div>]] | |||
<div align=left> | |||
==Appendix E: Seawater Composition=== | |||
[[File:Watson_seawater_appE.JPG|frame|center|border|<div align=center> <div>]] | |||
<div align=left> | |||
===Appendix F: Calculations for Projected Production=== | |||
[[File:Watson_appF.JPG|frame|center|border|<div align=center> <div>]] | |||
<div align=left> | |||
===Appendix G: Stream Table=== | |||
{| class="wikitable" style="margin: 1em auto 1em auto;" | |||
|+ '''Stream Table based on ASPEN model''' | |||
! Date: | |||
!Temperature (C) | |||
!Pressure (bar) | |||
!Vapor Frac | |||
!Solid Frac | |||
!Mole Flow (kmol/hr) | |||
!Mass Flow (kg/hr) | |||
!Volume Flow (cum/hr) | |||
!Enthalpy (Gcal/hr) | |||
!Mass Flow H2O (kg/hr) | |||
!Mass Flow NACL (kg/hr) | |||
!Mole Flow H2O (kmol/hr) | |||
!Mole Flow NACL (kmol/hr) | |||
|- | |||
| BR1 | |||
| 94.9 | |||
| 0.796 | |||
| 0 | |||
| 0 | |||
| 947915.803 | |||
| 19242000 | |||
| 24980.85 | |||
| -64524.976 | |||
| 16112200 | |||
| 3129820 | |||
| 894361.972 | |||
| 53553.831 | |||
|- | |||
| BR2 | |||
| 91.9 | |||
| 0.71 | |||
| 0 | |||
| 0 | |||
| 942373.503 | |||
| 19142200 | |||
| 24834.029 | |||
| -64207.75 | |||
| 16012300 | |||
| 3129820 | |||
| 888819.672 | |||
| 53553.831 | |||
|- | |||
| BR3 | |||
| 88.8 | |||
| 0.632 | |||
| 0 | |||
| 0 | |||
| 936866.23 | |||
| 19042900 | |||
| 24689.08 | |||
| -63892.397 | |||
| 15913100 | |||
| 3129820 | |||
| 883312.4 | |||
| 53553.831 | |||
|- | |||
| BR4 | |||
| 85.8 | |||
| 0.562 | |||
| 0 | |||
| 0 | |||
| 931429.146 | |||
| 18945000 | |||
| 24546.917 | |||
| -63580.935 | |||
| 15815200 | |||
| 3129820 | |||
| 877875.315 | |||
| 53553.831 | |||
|- | |||
| BR5 | |||
| 82.7 | |||
| 0.498 | |||
| 0 | |||
| 0 | |||
| 926025.475 | |||
| 18847600 | |||
| 24406.569 | |||
| -63271.259 | |||
| 15717800 | |||
| 3129820 | |||
| 872471.644 | |||
| 53553.831 | |||
|- | |||
| BR6 | |||
| 79.7 | |||
| 0.441 | |||
| 0 | |||
| 0 | |||
| 920689.773 | |||
| 18751500 | |||
| 24268.92 | |||
| -62965.351 | |||
| 15621700 | |||
| 3129820 | |||
| 867135.942 | |||
| 53553.831 | |||
|- | |||
| BR7 | |||
| 76.6 | |||
| 0.389 | |||
| 0 | |||
| 0 | |||
| 915386.001 | |||
| 18656000 | |||
| 24133.036 | |||
| -62661.146 | |||
| 15526200 | |||
| 3129820 | |||
| 861832.17 | |||
| 53553.831 | |||
|- | |||
| BR8 | |||
| 73.6 | |||
| 0.342 | |||
| 0 | |||
| 0 | |||
| 910148.127 | |||
| 18561600 | |||
| 23999.778 | |||
| -62360.597 | |||
| 15431800 | |||
| 3129820 | |||
| 856594.296 | |||
| 53553.831 | |||
|- | |||
| BRINE | |||
| 70.8 | |||
| 0.304 | |||
| 0 | |||
| 0 | |||
| 905441.542 | |||
| 18476800 | |||
| 23880.836 | |||
| -62090.445 | |||
| 15347000 | |||
| 3129820 | |||
| 851887.711 | |||
| 53553.831 | |||
|- | |||
| D1 | |||
| 94.9 | |||
| 0.796 | |||
| 1 | |||
| 0 | |||
| 3306.022 | |||
| 59558.904 | |||
| 126413.546 | |||
| -189.255 | |||
| 59558.904 | |||
| 0 | |||
| 3306.022 | |||
| 0 | |||
|- | |||
| D2 | |||
| 91.9 | |||
| 0.71 | |||
| 1 | |||
| 0 | |||
| 5542.299 | |||
| 99846.076 | |||
| 235561.239 | |||
| -317.403 | |||
| 99846.076 | |||
| 0 | |||
| 5542.299 | |||
| 0 | |||
|- | |||
| D3 | |||
| 88.8 | |||
| 0.632 | |||
| 1 | |||
| 0 | |||
| 5507.273 | |||
| 99215.065 | |||
| 260816.407 | |||
| -315.529 | |||
| 99215.065 | |||
| 0 | |||
| 5507.273 | |||
| 0 | |||
|- | |||
| D4 | |||
| 85.8 | |||
| 0.562 | |||
| 1 | |||
| 0 | |||
| 5437.085 | |||
| 97950.605 | |||
| 287426.924 | |||
| -311.637 | |||
| 97950.605 | |||
| 0 | |||
| 5437.085 | |||
| 0 | |||
|- | |||
| D5 | |||
| 82.7 | |||
| 0.498 | |||
| 1 | |||
| 0 | |||
| 5403.671 | |||
| 97348.64 | |||
| 319693.064 | |||
| -309.852 | |||
| 97348.64 | |||
| 0 | |||
| 5403.671 | |||
| 0 | |||
|- | |||
| D6 | |||
| 79.7 | |||
| 0.441 | |||
| 1 | |||
| 0 | |||
| 5335.702 | |||
| 96124.173 | |||
| 353955.3 | |||
| -306.082 | |||
| 96124.173 | |||
| 0 | |||
| 5335.702 | |||
| 0 | |||
|- | |||
| D7 | |||
| 76.6 | |||
| 0.389 | |||
| 1 | |||
| 0 | |||
| 5303.771 | |||
| 95548.926 | |||
| 395584.927 | |||
| -304.379 | |||
| 95548.926 | |||
| 0 | |||
| 5303.771 | |||
| 0 | |||
|- | |||
| D8 | |||
| 73.6 | |||
| 0.342 | |||
| 1 | |||
| 0 | |||
| 5237.874 | |||
| 94361.766 | |||
| 440141.36 | |||
| -300.723 | |||
| 94361.766 | |||
| 0 | |||
| 5237.874 | |||
| 0 | |||
|- | |||
| FEED | |||
| 11 | |||
| 1.013 | |||
| 0 | |||
| 0 | |||
| 56107.895 | |||
| 1032950 | |||
| 1083.597 | |||
| -3857.463 | |||
| 1000930 | |||
| 32021.357 | |||
| 55559.983 | |||
| 547.912 | |||
|- | |||
| HOTFEED | |||
| 57.7 | |||
| 1.013 | |||
| 0 | |||
| 0 | |||
| 56107.895 | |||
| 1032950 | |||
| 1100.165 | |||
| -3810.03 | |||
| 1000930 | |||
| 32021.357 | |||
| 55559.983 | |||
| 547.912 | |||
|- | |||
| INPUT | |||
| 98 | |||
| 1.013 | |||
| 0 | |||
| 0 | |||
| 951221.824 | |||
| 19301600 | |||
| 25084.412 | |||
| -64714.056 | |||
| 16171700 | |||
| 3129820 | |||
| 897667.993 | |||
| 53553.831 | |||
|- | |||
| M1 | |||
| 70.7 | |||
| 0.304 | |||
| 0.004 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 402879.11 | |||
| -65216.53 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| M2 | |||
| 70.7 | |||
| 0.304 | |||
| 0.01 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 897392.279 | |||
| -65163.387 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| M3 | |||
| 70.7 | |||
| 0.304 | |||
| 0.015 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 1393370 | |||
| -65110.087 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| M4 | |||
| 70.8 | |||
| 0.304 | |||
| 0.021 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 1894150 | |||
| -65056.274 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| M5 | |||
| 70.8 | |||
| 0.304 | |||
| 0.027 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 2396470 | |||
| -65002.297 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| M6 | |||
| 70.8 | |||
| 0.304 | |||
| 0.032 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 2903710 | |||
| -64947.793 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| M7 | |||
| 70.8 | |||
| 0.304 | |||
| 0.038 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 3412590 | |||
| -64893.114 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| MIXED | |||
| 70.1 | |||
| 0.304 | |||
| 0 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 24740.57 | |||
| -65269.173 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| P1 | |||
| 93.4 | |||
| 0.796 | |||
| 0 | |||
| 0 | |||
| 3306.022 | |||
| 59558.904 | |||
| 61.859 | |||
| -221.768 | |||
| 59558.904 | |||
| 0 | |||
| 3306.022 | |||
| 0 | |||
|- | |||
| P2 | |||
| 90.3 | |||
| 0.71 | |||
| 0 | |||
| 0 | |||
| 5542.299 | |||
| 99846.076 | |||
| 103.478 | |||
| -372.082 | |||
| 99846.076 | |||
| 0 | |||
| 5542.299 | |||
| 0 | |||
|- | |||
| P3 | |||
| 87.3 | |||
| 0.632 | |||
| 0 | |||
| 0 | |||
| 5507.273 | |||
| 99215.065 | |||
| 102.608 | |||
| -370.033 | |||
| 99215.065 | |||
| 0 | |||
| 5507.273 | |||
| 0 | |||
|- | |||
| P4 | |||
| 84.3 | |||
| 0.562 | |||
| 0 | |||
| 0 | |||
| 5437.085 | |||
| 97950.605 | |||
| 101.092 | |||
| -365.615 | |||
| 97950.605 | |||
| 0 | |||
| 5437.085 | |||
| 0 | |||
|- | |||
| P5 | |||
| 81.2 | |||
| 0.498 | |||
| 0 | |||
| 0 | |||
| 5403.671 | |||
| 97348.64 | |||
| 100.269 | |||
| -363.664 | |||
| 97348.64 | |||
| 0 | |||
| 5403.671 | |||
| 0 | |||
|- | |||
| P6 | |||
| 78.2 | |||
| 0.441 | |||
| 0 | |||
| 0 | |||
| 5335.702 | |||
| 96124.173 | |||
| 98.815 | |||
| -359.382 | |||
| 96124.173 | |||
| 0 | |||
| 5335.702 | |||
| 0 | |||
|- | |||
| P7 | |||
| 75.2 | |||
| 0.389 | |||
| 0 | |||
| 0 | |||
| 5303.771 | |||
| 95548.926 | |||
| 98.036 | |||
| -357.522 | |||
| 95548.926 | |||
| 0 | |||
| 5303.771 | |||
| 0 | |||
|- | |||
| P8 | |||
| 72.1 | |||
| 0.342 | |||
| 0 | |||
| 0 | |||
| 5237.874 | |||
| 94361.766 | |||
| 96.639 | |||
| -353.366 | |||
| 94361.766 | |||
| 0 | |||
| 5237.874 | |||
| 0 | |||
|- | |||
| P9 | |||
| 69.4 | |||
| 0.304 | |||
| 0 | |||
| 0 | |||
| 4706.586 | |||
| 84790.457 | |||
| 86.695 | |||
| -317.757 | |||
| 84790.457 | |||
| 0 | |||
| 4706.586 | |||
| 0 | |||
|- | |||
| PREHEAT | |||
| 70.8 | |||
| 0.304 | |||
| 0.041 | |||
| 0 | |||
| 952338.165 | |||
| 19322900 | |||
| 3716880 | |||
| -64860.601 | |||
| 16191300 | |||
| 3131550 | |||
| 898754.695 | |||
| 53583.47 | |||
|- | |||
| PURE | |||
| 72.1 | |||
| 0.342 | |||
| 0.019 | |||
| 0 | |||
| 35737.995 | |||
| 643829.982 | |||
| 58727.526 | |||
| -2404.05 | |||
| 643829.982 | |||
| 0 | |||
| 35737.995 | |||
| 0 | |||
|- | |||
| TANK | |||
| 70.1 | |||
| 0.304 | |||
| 0 | |||
| 0 | |||
| 961549.437 | |||
| 19509800 | |||
| 24979.868 | |||
| -65900.475 | |||
| 16347900 | |||
| 3161840 | |||
| 907447.693 | |||
| 54101.743 | |||
|- | |||
| WASTE | |||
| 70.1 | |||
| 0.304 | |||
| 0 | |||
| 0 | |||
| 9211.272 | |||
| 186896 | |||
| 239.297 | |||
| -631.301 | |||
| 156606.799 | |||
| 30289.201 | |||
| 8692.998 | |||
| 518.274 | |||
|} | |||
=References= | =References= | ||
1. Global Agenda Council on Water. World Economic Forum website. http://www.weforum.org/communities/global-agenda-council-on-water. Accessed January 14, 2016. | |||
"Calculated demand for water and energy on basis of population growth." | |||
2. Desalination industry enjoys growth spurt as scarcity starts to bite. Global Water Intelligence website. https://www.globalwaterintel.com/desalination-industry-enjoys-growth-spurt-scarcity-starts-bite/. Accessed January 14, 2015. | |||
"Study about desalination plants and their effectiveness in different regions." | |||
3. Sieder, Everett N, inventor; Us Interior, assignee. Multistage flash distillation with scale removal. US patent 3,476,654. November 4, 1969. | |||
"Patent on multistage flash distillation." | |||
4. Lee, KP, Arnot, TC, Mattia, D. A review of reverse osmosis membrane materials for desalination - Development to date and future potential. Journal of Membrane Science. 2011, 370: 1-22. | |||
"Article discussing efficacy of materials in reverse osmosis membranes." | |||
5. House, Kelley. Oregon drought forces cities to impose water use cutbacks. The Oregonian. http://www.oregonlive.com/environment/index.ssf/2015/08/oregon_drought_forces_cities_t.htm. Published August 1, 2015. Accessed January 13, 2016. | |||
"News article about severity of Oregon droughts." | |||
6. Oregon State Profile and Energy Estimates. U.S. Energy Information Administration. http://www.eia.gov/state/?sid=OR. Updated October 15, 2015. Accessed January 13, 2016. | |||
"Government study of Oregon’s energy and water needs." | |||
7. Batten, Belinda. Newport selected as home of Pacific Marine Energy Center. Oregon State University. http://oregonstate.edu/ua/ncs/archives/2013/jan/newport-selected-home-pacific-marine-energy-center. Published January 14, 2013. Accessed January 13, 2016. | |||
"Wave energy discussion by Oregon State professors." | |||
8. Oregon Agricultural Regions. State of Oregon Department of Agriculture. http://www.oregon.gov/ODA/shared/Documents/Publications/Administration/ORGrowingRegions.pdf. Accessed January 13, 2016. | |||
"Agricultural regions of Oregon." | |||
9. Salinity Distribution at the Ocean Surface. Centre Aval de Traitment des Données SMOS. http://www.salinityremotesensing.ifremer.fr/sea-surface-salinity/salinity-distribution-at-the-ocean-surface. Accessed January 14, 2016. | |||
"Tabulated data about salinity of ocean water in different regions." | |||
10. Maximum Contaminant Levels and Action Levels. Oregon Public Health Division. https://public.health.oregon.gov/HealthyEnvironments/DrinkingWater/Rules/Documents/61-0030.pdf. Published May 8, 2014. Accessed January 14, 2016. | |||
"Public mandates regarding maximum contaminant levels for potable water." | |||
11. Desalination and Water Recycling. Terrascope. http://12.000.scripts.mit.edu/mission2017/desalination-and-water-recycling/. Accessed January 13, 2016. | |||
"Cost-benefit analysis of desalination and other forms of water recycling." | |||
12. Cath, T.Y., Childress, A.E., Elimelech, M. Forward osmosis: Principles, applications, and recent developments. Journal of Membrane Science. 2006. 281: 70-87. | |||
"Summary of the current state of forward osmosis technology." | |||
13. Greenlee, L.F., Lawler, D.F., Freeman, B.D., Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Research. 2009. 43: 2317-2348. | |||
"Summary of the current state of reverse osmosis technology." | |||
14. Maximum Contaminant Levels and Action Levels. Oregon Health Authority website. http://public.health.oregon.gov/HealthyEnvironments/DrinkingWater/Rules/Documents/61-0030.pdf. Accessed January 28, 2016. | |||
"Oregon drinking water maximum contamination limits." | |||
15. Winters, H. Twenty years experience in sea water reverse osmosis and how chemicals in pretreatment affect fouling of membranes. Desalination. 1997. 110: 93-96. | |||
"Fouling of membranes in reverse osmosis." | |||
16. Refrigeration Cycles. Oklahoma University. http://www.ou.edu/class/che-design/che5480-07/Refrigeration%20Basics%20and%20LNG.pdf. Accessed January 27, 2016. | |||
Details on selection of appropriate refrigerant. | |||
17. Williamson, William R, inventor; American Mach & Foundry, assignee. Multistage flash distillation apparatus. U.S. patent 3,399,118. August 27, 1968. | |||
"Basis for our design. MSF with connected chambers, one eductor, and a complete brine dilution recycle." | |||
18. El-Dessouky, H.T., Ettouney, H.M., Al-Roumi, Y. Multi-stage flash desalination: present and future outlook. Chemical Engineering Journal. 1999, 73: 173-190. | |||
"Summary of MSF processes both traditional, and a new recycle method." | |||
19. Kaghazchi, Tahereh, et al. "A mathematical modeling of two industrial seawater desalination plants in the Persian Gulf region." Desalination 252.1 (2010): 135-142. Accessed February 29, 2016. | |||
"Temperature and pressure profiles through multiple connected flash chambers." | |||
20. Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013. | |||
"Price comparison between carbon steel and stainless steel; equipment sizing" | |||
21. Malik, A. U., Al-Fozan, S. A. Corrosion and materials selection in MSF desalination plants. Corrosion Reviews. 2011: 29: 153-175. | |||
"Material performance for MSF in the presence of saltwater" | |||
22. Ghani, S., Al-Deffeeri, N. S. Impacts of different antiscalant dosing rates and their thermal performances in Multi Stage Flash (MSF) distiller in Kuwait. Desalination. 2010: 250: 463-472. | |||
"Background information on scaling" | |||
23. Scaling and Antiscalants. Lenntech Water Treatment Solutions website. http://www.lenntech.com/antiscalants.htm. Accessed February 28, 2016. | |||
"Scalant information and pricing" | |||
24. Utility Bill Calculator. City of Newport, Oregon website. http://www.thecityofnewport.net/dept/pwk/billcalc.asp. Accessed February 25, 2016. | |||
"Price of water in Newport, Oregon" | |||
25. Statewide Water Needs Assessment Oregon Water Supply and Conservation Initiative. Oregon Water Resources Department. http://www.oregon.gov/owrd/law/docs/owsci/owrd_demand_assessment_report_final_september_2008.pdf. Accessed January 14, 2016. | |||
"Optimization study about increasing water supply and demand chain problems." | |||
Latest revision as of 18:16, 11 March 2016
Team G: Newport Desalination Plant
Authors: KC Anderson, Neil Dalvie, Watson Fu, Helen Wu
Instructors: Fengqi You, David Wegerer
March 11, 2016
Executive Summary
This reports outlines the design and evaluation of a multi-stage flash distillation plant located in Newport, Oregon. As the human population increases, so does the need for new sources of freshwater. Global water use is currently at 9 trillion cubic meters per year and is expected to increase by about 60 billion cubic meters per year. In addition, droughts in the west coast of the United States have made the need for new sources of freshwater much more urgent in recent years. Oregon state recently announced the end of drought conditions from the last few years. This plant is designed as preemptive action to reduce the effects of future droughts by meeting ~10% of the expected increase in water demand in the mid-coastal region of Oregon. However, prohibitive costs in the current design may inhibit preemptive investment.
The choice of an MSF process over membrane technologies was twofold. First, Oregon has strict water purity limits that can be more easily met with a robust thermal separation. Second, Newport is a hub of renewable energy research, and we believe that in the future this process can be paired with cheap, renewable thermal energy. The process consists of a major 9 stage flash vacuum unit, and a large surge tank. Feed and recycle are mixed in the surge tank for heat capture, and flows are optimized for a 62% seawater yield. The flash unit consists of 9 conjoined drums with condensing equipment and collection trays in the upper portions. Seawater feed is used as the cooling medium for condensation.
Optimization for high yield of distillate has the unwanted effect of creating large flows through the flash unit recycle loop. These flows mandate nearly unrealistic heat transfer requirements, resulting in large equipment and prohibitively high utility requirements. In future iterations of this design, we recommend parallel processes with a reduced distillate yield. Flash situations with lower flow rates and energies are essential for an affordable process.
An economic analysis revealed a loss of several billion dollars in a 20 year prediction. This was largely due to a high utility requirement. The predicted net present value at 20 years is most sensitive to heat and power requirements. With a refined design and committed investment and government subsidies, this process may be feasible, while expensive. However, given the current water climate and price, an investment of this magnitude may be difficult to accomplish in preemptive non-drought conditions. Therefore, we recommend a refined process design, and reevaluation of the water market and availability in Oregon in 3-5 years.
Introduction
As the human population increases, so does the need for new sources of freshwater. Global water use is currently at 9 trillion cubic meters per year and is expected to increase by about 60 billion cubic meters per year. In addition, droughts in the west coast of the United States have made the need for new sources of freshwater much more urgent in recent years. Growing urban populations in developed countries also have high requirements for water, and 39% of global population lives within 100 kilometers of an ocean coast.1 This means that a large percentage of people do not have access to fresh water sources. Because of these concerns, a large market exists for desalinated water. As of 2013, desalination plants produced 78.4 million cubic meters of water per day and this number is expected to increase.2
There are two main categories of methods of desalination used in industry. The first category is thermal-based separation. Multi-Stage Flash Distillation (MSF) has been widely utilized and involves heating and pressurizing impure water to separate water vapor. MSF is the most popular thermal separation method because of the high purity that can be obtained.3 The second category is membrane-based separation. Reverse Osmosis (RO) is becoming the preferred method in industry. RO uses a pressure gradient to drive water through a membrane. Compared to most other methods, RO has low energy requirements and higher yield.4
The purpose of this report is to examine the potential implementation of a MSF desalination plant and evaluate the economic feasibility of the design. The remainder of the report outlines the process design, economics of the design, and important recommendations to further optimize the design and increase economic feasibility.
Design Basis
Location
This desalination plant will be located in Newport, Oregon to provide water to the mid-coastal region of Oregon. Oregon has recently suffered a major drought, and 23 out of 36 counties implemented agricultural water regulation and applied for federal assistance.5 As 2016 arrives, Oregon has ended its state drought emergency, but many new water regulations and conservation efforts appear to be permanent going forward. Oregon also offers an abundance of and commitment to clean energy not seen in other states. In California, desalination in reaction to extreme drought has forced plants to rely on destructive fossil fuel energy sources, thereby offsetting the environmental effect of desalination. A desalination process in Oregon would be posed to take advantage of a high and growing grid of renewable power. So far, 73% of Oregon’s electricity generation is renewable.6 The town of Newport boasts proximity to free coastline, and separation from major wildlife and forest reserves. In 2013, Oregon State University selected Newport as the location for its Pacific Marine Energy Center, a large scale trial of renewable wave energy.7 Independent of the efficacy of wave energy, this project indicates the overall availability and commitment to renewable energy in Newport. Finally, while it is slightly farther from the drought stricken southern Oregon counties that are most affected by the California shortage, Newport lies in proximity to the agriculture-rich and highly water-dependent agriculture in the Willamette valley.8
Process Requirements
This plant will produce 15,500 cubic meters of desalinated water per day, aiming to offset on the order of ~10% of predicted increase in out-of-stream water demand in the mid-coastal region in coming years. The feed for this process is only seawater, sourced from the Newport coastal water with a salinity of 32 PSU (g/kg of seawater).9 The process will produce 99.2% desalinated, potable water with a maximum chloride concentration of 250 mg/L and total dissolved solids of 500 mg/L. As waste, the process will release diluted, cooled, brine from a surge tank. Composition of the feed seawater can be found in Appendix E.
Technical Approach
We decided to use Multi-Stage Flash Distillation (MSF) for the desalination process in our plant. Principally, MSF allows us to achieve the purity required for Oregon regulations. Oregon water regulations include an upper limit of salt concentration at 250 mg/L for potable water.10 Because of this, MSF provides a more reliable high purity product than does reverse osmosis, the main alternative. In addition, thermal methods like MSF achieve the desired purity with less dependence on input conditions. While we expect seawater concentrations to remain largely constant, a robust process is desirable. While membranes require significant pretreatment of feeds, thermal methods can process raw seawater and do not run the risk of microbial contamination.4 Despite this advantage, MSF typically sees considerably lower yield, and higher thermal energy costs than reverse osmosis.11 This decision was made after considering a number of options, described in this section. Design alternatives are based on a simple separation block diagram, shown in Appendix A.
Process Alternatives
Pressure Control Design Options
An important aspect of most desalination processes is establishing a pressure gradient. In membrane technologies, the pressure gradient is a driving force for separation against a concentration gradient. Forward osmosis holds a major advantage in this section of the process, as little to no gauge pressure is required to drive osmosis.12 In comparison, reverse osmosis requires high levels of pressure to achieve separation.13 The magnitude of the pressures increases capital costs and utilities costs tremendously, which is a significant disadvantage. For thermal separation technology, low pressure works in accordance with the thermal changes to remove steam from the concentrated brine, as the water vapor saturation temperature changes with changing pressure. The two main methods of vacuum creation are seawater eductors and vacuum pumps. An eductor is convenient when high energy flows are accessible within the process. In the absence of extra flows, we decided to utilize a simple vacuum pump. While energy intensive, this pump achieves low pressures easily.
Pretreatment Design Options
Membrane technologies, including forward and reverse osmosis, are limited by the size and selectivity of the membrane. This presents an issue, as Oregon mandates strict upper limits on organic contaminants.14 One solution to this issue is to source water from either several hundred meter depth or from beach wells, where water has already passed through sediment.15 In addition to feed requirements, reverse osmosis methods require several pretreatment steps to avoid severe membrane fouling.13 Forward osmosis processes require the addition of a draw solution on the permeate side of the membrane to create an osmotic pressure driving force.12 Thermal desalination relies on the heating of seawater to obtain a pure distillate. In early design stages, we considered the implementation of a refrigeration loop. Unfortunately, the purchase of refrigerants are prohibitively expensive,16 and a refrigeration loop is beneficial when heat needs to be transferred from one area of the process to another. With the implementation of a vacuum pump, there is nothing in the process that needs to be cooled. For this reason and cost, we decided to heat our process stream using a condensing steam heat exchanger.
Separation Design Options
One of the main separation methods for desalination is membrane separation. Forward osmosis relies on a membrane to allow transfer of water under purely osmotic forces. However, continuous flow is difficult to arrange spatially since the concentrated draw solution must be recycled back through the system. Very little literature exists on practical uses of forward osmosis membranes for desalination, so we have chosen to avoid this option. Reverse osmosis uses hydraulic pressure to force osmosis, rather than a draw solution and concentration gradient. Reverse osmosis can generally achieve only 98% salt removal, requiring multiple passes.12 A vast majority of MSF processes are centered around a series of flash chambers with descending pressure and temperature. Vaporized water is collected in a tray as the pure distillate, with increasingly concentrated brine flowing into the next flash chamber. In order to maintain the pressure gradient needed, a vacuum pump is used. By aligning the flash chambers into one unit, only one pump would be needed to create the pressure gradient, reducing both capital and operating costs.17 Therefore, we have decided to move forward with MSF with the use of flash chambers connected into one unit for our separation.
Waste Treatment Design Options
Reverse osmosis typically requires additional steps to return the water product to an acceptable pH after the initial acidification before release, in addition to dilution.13 Forward osmosis technology requires separation of pure water from the draw solution through heating. This adds significantly to the otherwise minimal energy requirement of a forward osmosis process.12 One technology that could improve waste treatment for an MSF process is adding a brine recycle. Two methods of concentrated brine recycle are prevalent. In one method, a portion of concentrated brine is recycled into the seawater feed, with the rest of the brine sent to dilution and waste.18 Alternatively, concentrated bring can be rerouted to a surge tank. This tank is controlled to maintain a concentration acceptably diluted for waste, serving as the seawater feed and the waste “purge”, with the two having the same composition.17 Traditionally in chemical processes, recycle systems require more energy to carry out the process. Because desalination is itself a separation, recycle may be advantageous because of the retained heat energy. In the second recycle method, the surge tank serves not only to cool the diluted waste to an acceptable release temperature, but also to preheat the process feed. In this setup, where no heat is rejected into the waste, thermal efficiency may actually increase, decreasing utility costs.18 For these reasons, we have decided to implement a surge tank recycle stream.
Results
Design Tradeoffs and Process Optimization
Once the overall design equipment and strategy was selected, mass and energy balances were calculated and optimized for yield and cost. To determine these values, temperatures, and flow rates, we made a number of assumptions and set points in our process. The feed and waste concentrations were held constants, at the composition of Oregon sea water, and the maximum allowable waste concentration. The flash inlet was held at 1 atm and 98°C, in order to maximize energy carried by the stream without premature boiling. The distillate flow rate was held constant in line with our initial problem statement and project goals. Finally, phase data was obtained from Aspen+. While true seawater will contain other contaminants, these have small effects on thermodynamic properties. Pretreatment and material selection will take additional contaminants into consideration, but they are neglected in mass and energy calculations.
Pressure Considerations and Yield
Aspen+ phase data revealed that because the energy used to vaporize the water is carried in the inlet stream, the amount of water flashed depends almost completely on the pressure in the last flash stage, or the lowest pressure in the process. Because of this, the mass balances over the entire process are largely dependent on the equilibrium conditions in the last drum. Therefore, for overall balances, we treated the connected series of flash drums as one unit. This assumption is based on the adiabatic nature of the drums, and the assumption that the brine reaches phase equilibrium before leaving the unit. This yields a simplified block diagram for the purpose of calculating overall mass balances, as shown in Appendix A. Figure 1a shows conditions at a range of vacuum pressures. As pressure is decreased, the yield of vaporization increases, which corresponds to an increase in the outlet concentration of NaCl for recycle. Temperature decreases with pressure to maintain vapor-liquid saturation conditions. The temperature profile is critical in designing the multistage flash unit, as higher temperatures through the pressure gradient will release hot distillate that can be captured in preheating.

At first inspection, it appears advantageous to operate at the lowest possible pressure to obtain the highest vaporization yield. However, dilution for waste proved to be a more significant factor in overall process yield than the yield over the flash drum unit. Operating at the lowest possible pressure maximizes vapor yield, but creates a more concentrated recycle stream. This higher concentration requires more process feed to dilute to waste conditions, lowering the overall process yield. For this reason, it is desirable to produce a recycle stream as close to waste concentration as possible, minimizing the amount of process feed needed to dilute to waste conditions. Figure 1b shows the effect of flash pressure on overall process metrics. It becomes clear that the overall yield increases with pressure as an asymptote. Above a certain pressure, the recycle stream becomes too dilute to create a waste concentration of 40 g/kg, creating a negative feed requirement for this calculation. Because we would like to release waste of 40 g/kg, we focus on the feasible solutions below 0.4 atm. Figure 1b also shows the small effect on heating requirements as the pressure is changed. Because the amount of water vaporized is held constant, this energy is largely representative of the energy needed to vaporize that amount of water. Figure 1a shows that at higher operating pressures and lower vaporization yields, the brine recycle will remain hot. Therefore, despite increased recycle rates, the higher temperature keeps the energy requirement nearly constant. With these considerations, we will operate at a flash pressure that limits the vaporization yield, keeping the recycle stream near waste concentrations. When operating at a pressure of 0.3 atm, an overall yield of approximately 62% can be achieved. This higher pressure will also provide energy savings in vacuum creation.
Flash Stage Optimization and Sizing
The flash unit, where all flash stages occur, and makes up the bulk of the process. It consists of 9 vertical flash drums connected in series, each with a condenser in the upper portion. The drums are held at low pressure, allowing the volume to fill with saturated water vapor. This vapor condenses on heat exchange pipes in the top of the drum, and condenses, falling onto a collection tray. Once overall mass balances were calculated, detailed mass and energy balances on the major flash unit were analyzed. First, the equilibrium in each stage was characterized. Connected equilibrium stages exhibit linearly decreasing temperature.19 Optimization of mass balances called for a pressure of 0.3 atm in the last drum to achieve the highest yield. This produces the following equilibrium conditions across all 9 drums (the number of drums eventually selected).

Interestingly, this equilibrium profile creates nearly constant vapor flow rates in each drum. These flow rates, along with the density of saturated vapor in each drum, were used to calculate the minimum chamber diameter to allow enough vapor-liquid interface. The minimum diameter for the lowest pressure drum was approximately 6 m, and the minimum for preceding drums varied minimally. For manufacturing simplicity, we have decided to build all 9 drums using a diameter and height of 6 m. As explained previously, drums will be constructed of stainless steel to prevent corrosion. Because each drum will be operated in vacuum conditions, the pressure on drum walls will never exceed 1 atm. We therefore calculated the thickness of material based on structural feasibility, requiring a thickness of 3 cm. Pricing of the drums was approximated using the required weight of stainless steel. In addition to drum material, the cost of each condensing unit was approximated using the required heat exchange area to condense the distillate. This calculation was performed under the assumption that the surge tank outlet (See Appendix L) will be used as the cooling stream on the tube side. Approximate cost of each condensing system was derived heuristically from the heat transfer area. All of these equilibrium and costing calculations were repeated for a growing number of stages. Figure 2b indicates the total cost of the flash unit for different numbers of stages. Using this, we selected 9 tanks, within the region of diminishing returns. Once this number was selected, we began specific design of each condensing region of the drums. Notably, the last three drums require excessive heat transfer area that exceeds 5000 square meters. The last drum, which requires nearly 19000 square meters, can be remedied by using the process seawater feed to cool, which is at a significantly lower temperature than the surge tank. Unfortunately, the process feed does not have the heat capacity to cool the 7th and 8th drums. For this initial design and economic analysis, these drums retain an unrealistic heat transfer area. In implementation, additional cooling water would be required. The cost of demisters and trays were also considered. The approximate price for a 6-meter diameter demister is $500. Both the demister and condensing tray price were considered negligible compared to the overall price of the flash chambers.
Process Overview
The final design process flow diagram is shown in Appendix B. Feed seawater is pumped into the plant, and immediately used as a condensing sink in the last drum. The warmed seawater is then sent for mixing in the surge tank. The surge tank outlet is used as the condensing heat sink for the other 8 tanks in series, before being delivered to the heat exchanger. In the exchanger, the flash feed is heated to 98°C before entering the first drum. The brine then passes through all drums, reaching phase equilibrium in each one as the pressure is reduced. From the last drum, the concentrated brine is pumped out and back into the surge tank. The surge tank includes a waste purge back out to the ocean. Stream tables are included with compositions and conditions for each stream. Notably, the concentration in the surge tank is 40 g/kg, the maximum allowable waste concentration. This also serves as the flash feed. In addition, there is a large amount of fluid in recirculation through the recycle loop, with relatively small process feed and waste. This has many implication, positive in the large increase in overall yield, and negative in the energy costs and large equipment sizes required. Economic implications of these large flows are addressed in later sections. For reference, the Aspen+ file used for phase and mass balance calculations is shown in Appendix C.
Equipment Sizing
Surge Tank
To account for corrosion, the surge mixing tank will be constructed of stainless steel. The size of the surge tank was based on two criteria. First, a residence time of 30 minutes was specified to ensure full mixing of process feed and brine recycle. Second, the tank is designed to hold a large percentage of the brine in recycle circulation, in case the process needs experiences a sudden shutdown. These criteria resulted in surge tank dimensions of a diameter and height of 24.3 m.
Heater
The heater E-101 is the primary energy input for the process. It uses condensing steam to heat the flash feed to 98 C. This heat exchange will be very large, measuring 25 ft. in length and 7 ft. in diameter. It requires high amount of high pressure steam, and results in a pressure drop of 25 bar, creating much of the pumping requirement for the process. Detailed design of the heater can be found in Appendix M.
Pumps
Based on sizing estimations given in Towler20, Ch. 7, P-101 will be $323,151, P-102 will be $12,446, and P-103 will be $311,369. The utilities can be calculated using the brake hp of the pumps. P-101 has a brake hp of 166,870 kW, P-102 has a brake hp of 4.1 kW, and P-103 has a brake hp of 457.37 kW. The utility cost for P-101 is large because of the pressure drop in the heater, E-101. Detailed design of pumps can be found in Appendices N-P.
Safety, Control, and Environmental Considerations
Controls
This process exhibits four major control loops. First, two control loops exist within the surge tank, comprising most of the process control. Liquid level is controlled by manipulating the waste flow rate, and composition is manipulated by controlling the feed flow rate. Pressure in the flash unit is controlled by manipulating the vacuum pump power. Finally, the brine inlet temperature is controlled by altering the steam delivered to the heat exchanger.
Environmental Considerations
Our process does not include a significant post treatment, and produces de-ionized water because our plant must meet demands for both agriculture and human consumption. Because Oregon has recently left drought conditions, we believe that local treatment centers currently have additional capacity available. Oregon has strict regulations on the salinity of wastewater for marine health. Our process reaches but does not exceed the maximum salt concentration of 40g/L in wastewater. We plan to utilize clean energy for our process. Oregon offers an abundance of and commitment to clean energy not seen in other states. In California, desalination in reaction to extreme drought has forced plants to rely on destructive fossil fuel energy sources, thereby offsetting the environmental effect of desalination. A desalination process in Oregon would be posed to take advantage of a high and growing grid of renewable power. So far, 73% of Oregon’s electricity generation is renewable.6
Scaling and Corrosion
In the brine recycle stream, salt levels climb as high as 41 g/kg. While not extreme conditions, this salinity along with additional contaminants brings attention to the prevention of corrosion and control of scaling. We have decided to build our desalination plant with 316 stainless steel because of its excellent resistance to general and localized corrosion.21 Although 316 stainless steel is three times as expensive as carbon steel it will maintain efficient operation with less failures due to corrosion damage.20 We have decided to add an antiscalant to our process instead of acid addition.22 Acidification, although effective in preventing the precipitation of calcium carbonate, is relatively ineffective in preventing other types of scale and also less cost effective.23 Based on performance studies, we have decided to use a polyphosphate acid inhibitor as our antiscalant, at a dosing rate of 1.5ppm due to the relatively low maximum operating temperature of 98°C.22
Economic Evaluation and Sensitivity Analysis
The ISBL capital costs were estimated to be 14.5 MM$, while OSBL costs were estimated to be 40% of ISBL costs. Individual equipment costs can be found in Appendix D. Since the plant is located on the West Coast, a location factor of 1.07 was applied. The variable cost of production for the plant has three main sources: raw materials, consumables, and utilities. The main raw materials costs are from the antiscalant, since we will not have to pay for the seawater feed; the antiscalant will cost $542,000/year for the flow of 100,000 m3 of seawater per day. Utility costs mainly consisted of electricity for the pumps and steam for the heat exchanger. Detailed equipment and utility costs are shown in Appendix D. The total fixed capital cost was calculated to be 129.7 MM$. Major assumptions include having three shifts of five operators earning $50,000 salaries, maintenance of the plant at 5% of ISBL costs, and direct labor overhead being 25% of operator costs.
Profitability of the plant was assessed by calculating the net present value (NPV) and internal rate of return (IRR). The price of purified water was assumed to be $3.00 per 1000 gallons from average water costs in Newport, Oregon and assuming we will have to sell our water at a lower price to treatment facilities before being sold for public use.24 Assuming a 5-year MACRS depreciation schedule, this plant is not profitable by a large margin, losing up to 7 billion dollar over a 20 year window. Main sources of the imbalance are high utility costs ($150 MM for high pressure steam). If profit is the goal of this process, we would not recommend pursuing this project. However, if there a pressing need and investment for purified water, and a potential source of local energy from renewable sources, this process may be a feasible project, though expensive. The full economic analysis can be found in Appendix J. A sensitivity analysis revealed that lowering high energy requirements is imperative to drive down prohibitive costs. In addition, subsidies would be necessary to execute this process. Sensitivity to several process parameters is represented in Figure 3.
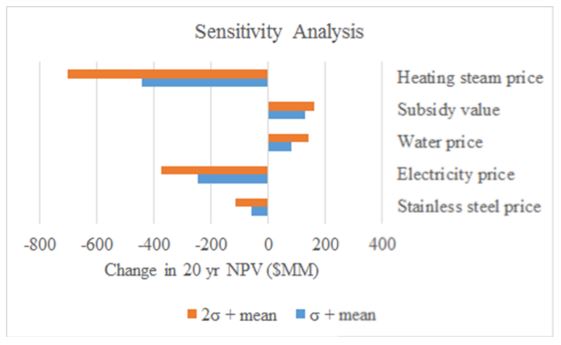
Conclusion
In this report we outline a proposed multi-stage flash distillation process designed to meet ~10% of the expected increase in mid-coastal Oregon water demand. The plant uses a 9 flash drum vacuum unit for distillation, and a large surge tank for heat capture and process control. Most major concerns with the current proposed plant involve the massive flow rates through the recycle loop. This creates large energy requirements and unrealistic heat exchange at several locations. In a refined design, we recommend sacrificing process yield to reduce the recycle ratio. A lower flash yield with several identical processes in parallel allows for reasonable heat exchange and equipment design. The abundance of cool seawater should be used more fully in the process.
An economic evaluation revealed significant losses in a 20 year prediction. With improved process design, this plant could be feasible with committed investment and government subsidies. However, this plant is designed to meet future needs, which are not pressing right now. Due to the immense energy intensive cost of this project, it may be difficult to secure support for a preemptive desalination strategy, and merits reevaluation in 3-5 years.
Appendices
Appendix A: Block Flow Diagram

Appendix B: Process flow diagram

Appendix C: Aspen Model=

Appendix D: Equipment and Utility Costs=


Appendix E: Seawater Composition=

Appendix F: Calculations for Projected Production

Appendix G: Stream Table
| Date: | Temperature (C) | Pressure (bar) | Vapor Frac | Solid Frac | Mole Flow (kmol/hr) | Mass Flow (kg/hr) | Volume Flow (cum/hr) | Enthalpy (Gcal/hr) | Mass Flow H2O (kg/hr) | Mass Flow NACL (kg/hr) | Mole Flow H2O (kmol/hr) | Mole Flow NACL (kmol/hr) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR1 | 94.9 | 0.796 | 0 | 0 | 947915.803 | 19242000 | 24980.85 | -64524.976 | 16112200 | 3129820 | 894361.972 | 53553.831 |
| BR2 | 91.9 | 0.71 | 0 | 0 | 942373.503 | 19142200 | 24834.029 | -64207.75 | 16012300 | 3129820 | 888819.672 | 53553.831 |
| BR3 | 88.8 | 0.632 | 0 | 0 | 936866.23 | 19042900 | 24689.08 | -63892.397 | 15913100 | 3129820 | 883312.4 | 53553.831 |
| BR4 | 85.8 | 0.562 | 0 | 0 | 931429.146 | 18945000 | 24546.917 | -63580.935 | 15815200 | 3129820 | 877875.315 | 53553.831 |
| BR5 | 82.7 | 0.498 | 0 | 0 | 926025.475 | 18847600 | 24406.569 | -63271.259 | 15717800 | 3129820 | 872471.644 | 53553.831 |
| BR6 | 79.7 | 0.441 | 0 | 0 | 920689.773 | 18751500 | 24268.92 | -62965.351 | 15621700 | 3129820 | 867135.942 | 53553.831 |
| BR7 | 76.6 | 0.389 | 0 | 0 | 915386.001 | 18656000 | 24133.036 | -62661.146 | 15526200 | 3129820 | 861832.17 | 53553.831 |
| BR8 | 73.6 | 0.342 | 0 | 0 | 910148.127 | 18561600 | 23999.778 | -62360.597 | 15431800 | 3129820 | 856594.296 | 53553.831 |
| BRINE | 70.8 | 0.304 | 0 | 0 | 905441.542 | 18476800 | 23880.836 | -62090.445 | 15347000 | 3129820 | 851887.711 | 53553.831 |
| D1 | 94.9 | 0.796 | 1 | 0 | 3306.022 | 59558.904 | 126413.546 | -189.255 | 59558.904 | 0 | 3306.022 | 0 |
| D2 | 91.9 | 0.71 | 1 | 0 | 5542.299 | 99846.076 | 235561.239 | -317.403 | 99846.076 | 0 | 5542.299 | 0 |
| D3 | 88.8 | 0.632 | 1 | 0 | 5507.273 | 99215.065 | 260816.407 | -315.529 | 99215.065 | 0 | 5507.273 | 0 |
| D4 | 85.8 | 0.562 | 1 | 0 | 5437.085 | 97950.605 | 287426.924 | -311.637 | 97950.605 | 0 | 5437.085 | 0 |
| D5 | 82.7 | 0.498 | 1 | 0 | 5403.671 | 97348.64 | 319693.064 | -309.852 | 97348.64 | 0 | 5403.671 | 0 |
| D6 | 79.7 | 0.441 | 1 | 0 | 5335.702 | 96124.173 | 353955.3 | -306.082 | 96124.173 | 0 | 5335.702 | 0 |
| D7 | 76.6 | 0.389 | 1 | 0 | 5303.771 | 95548.926 | 395584.927 | -304.379 | 95548.926 | 0 | 5303.771 | 0 |
| D8 | 73.6 | 0.342 | 1 | 0 | 5237.874 | 94361.766 | 440141.36 | -300.723 | 94361.766 | 0 | 5237.874 | 0 |
| FEED | 11 | 1.013 | 0 | 0 | 56107.895 | 1032950 | 1083.597 | -3857.463 | 1000930 | 32021.357 | 55559.983 | 547.912 |
| HOTFEED | 57.7 | 1.013 | 0 | 0 | 56107.895 | 1032950 | 1100.165 | -3810.03 | 1000930 | 32021.357 | 55559.983 | 547.912 |
| INPUT | 98 | 1.013 | 0 | 0 | 951221.824 | 19301600 | 25084.412 | -64714.056 | 16171700 | 3129820 | 897667.993 | 53553.831 |
| M1 | 70.7 | 0.304 | 0.004 | 0 | 952338.165 | 19322900 | 402879.11 | -65216.53 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| M2 | 70.7 | 0.304 | 0.01 | 0 | 952338.165 | 19322900 | 897392.279 | -65163.387 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| M3 | 70.7 | 0.304 | 0.015 | 0 | 952338.165 | 19322900 | 1393370 | -65110.087 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| M4 | 70.8 | 0.304 | 0.021 | 0 | 952338.165 | 19322900 | 1894150 | -65056.274 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| M5 | 70.8 | 0.304 | 0.027 | 0 | 952338.165 | 19322900 | 2396470 | -65002.297 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| M6 | 70.8 | 0.304 | 0.032 | 0 | 952338.165 | 19322900 | 2903710 | -64947.793 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| M7 | 70.8 | 0.304 | 0.038 | 0 | 952338.165 | 19322900 | 3412590 | -64893.114 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| MIXED | 70.1 | 0.304 | 0 | 0 | 952338.165 | 19322900 | 24740.57 | -65269.173 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| P1 | 93.4 | 0.796 | 0 | 0 | 3306.022 | 59558.904 | 61.859 | -221.768 | 59558.904 | 0 | 3306.022 | 0 |
| P2 | 90.3 | 0.71 | 0 | 0 | 5542.299 | 99846.076 | 103.478 | -372.082 | 99846.076 | 0 | 5542.299 | 0 |
| P3 | 87.3 | 0.632 | 0 | 0 | 5507.273 | 99215.065 | 102.608 | -370.033 | 99215.065 | 0 | 5507.273 | 0 |
| P4 | 84.3 | 0.562 | 0 | 0 | 5437.085 | 97950.605 | 101.092 | -365.615 | 97950.605 | 0 | 5437.085 | 0 |
| P5 | 81.2 | 0.498 | 0 | 0 | 5403.671 | 97348.64 | 100.269 | -363.664 | 97348.64 | 0 | 5403.671 | 0 |
| P6 | 78.2 | 0.441 | 0 | 0 | 5335.702 | 96124.173 | 98.815 | -359.382 | 96124.173 | 0 | 5335.702 | 0 |
| P7 | 75.2 | 0.389 | 0 | 0 | 5303.771 | 95548.926 | 98.036 | -357.522 | 95548.926 | 0 | 5303.771 | 0 |
| P8 | 72.1 | 0.342 | 0 | 0 | 5237.874 | 94361.766 | 96.639 | -353.366 | 94361.766 | 0 | 5237.874 | 0 |
| P9 | 69.4 | 0.304 | 0 | 0 | 4706.586 | 84790.457 | 86.695 | -317.757 | 84790.457 | 0 | 4706.586 | 0 |
| PREHEAT | 70.8 | 0.304 | 0.041 | 0 | 952338.165 | 19322900 | 3716880 | -64860.601 | 16191300 | 3131550 | 898754.695 | 53583.47 |
| PURE | 72.1 | 0.342 | 0.019 | 0 | 35737.995 | 643829.982 | 58727.526 | -2404.05 | 643829.982 | 0 | 35737.995 | 0 |
| TANK | 70.1 | 0.304 | 0 | 0 | 961549.437 | 19509800 | 24979.868 | -65900.475 | 16347900 | 3161840 | 907447.693 | 54101.743 |
| WASTE | 70.1 | 0.304 | 0 | 0 | 9211.272 | 186896 | 239.297 | -631.301 | 156606.799 | 30289.201 | 8692.998 | 518.274 |
References
1. Global Agenda Council on Water. World Economic Forum website. http://www.weforum.org/communities/global-agenda-council-on-water. Accessed January 14, 2016.
"Calculated demand for water and energy on basis of population growth."
2. Desalination industry enjoys growth spurt as scarcity starts to bite. Global Water Intelligence website. https://www.globalwaterintel.com/desalination-industry-enjoys-growth-spurt-scarcity-starts-bite/. Accessed January 14, 2015.
"Study about desalination plants and their effectiveness in different regions."
3. Sieder, Everett N, inventor; Us Interior, assignee. Multistage flash distillation with scale removal. US patent 3,476,654. November 4, 1969.
"Patent on multistage flash distillation."
4. Lee, KP, Arnot, TC, Mattia, D. A review of reverse osmosis membrane materials for desalination - Development to date and future potential. Journal of Membrane Science. 2011, 370: 1-22.
"Article discussing efficacy of materials in reverse osmosis membranes."
5. House, Kelley. Oregon drought forces cities to impose water use cutbacks. The Oregonian. http://www.oregonlive.com/environment/index.ssf/2015/08/oregon_drought_forces_cities_t.htm. Published August 1, 2015. Accessed January 13, 2016.
"News article about severity of Oregon droughts."
6. Oregon State Profile and Energy Estimates. U.S. Energy Information Administration. http://www.eia.gov/state/?sid=OR. Updated October 15, 2015. Accessed January 13, 2016.
"Government study of Oregon’s energy and water needs."
7. Batten, Belinda. Newport selected as home of Pacific Marine Energy Center. Oregon State University. http://oregonstate.edu/ua/ncs/archives/2013/jan/newport-selected-home-pacific-marine-energy-center. Published January 14, 2013. Accessed January 13, 2016.
"Wave energy discussion by Oregon State professors."
8. Oregon Agricultural Regions. State of Oregon Department of Agriculture. http://www.oregon.gov/ODA/shared/Documents/Publications/Administration/ORGrowingRegions.pdf. Accessed January 13, 2016.
"Agricultural regions of Oregon."
9. Salinity Distribution at the Ocean Surface. Centre Aval de Traitment des Données SMOS. http://www.salinityremotesensing.ifremer.fr/sea-surface-salinity/salinity-distribution-at-the-ocean-surface. Accessed January 14, 2016.
"Tabulated data about salinity of ocean water in different regions."
10. Maximum Contaminant Levels and Action Levels. Oregon Public Health Division. https://public.health.oregon.gov/HealthyEnvironments/DrinkingWater/Rules/Documents/61-0030.pdf. Published May 8, 2014. Accessed January 14, 2016.
"Public mandates regarding maximum contaminant levels for potable water."
11. Desalination and Water Recycling. Terrascope. http://12.000.scripts.mit.edu/mission2017/desalination-and-water-recycling/. Accessed January 13, 2016.
"Cost-benefit analysis of desalination and other forms of water recycling."
12. Cath, T.Y., Childress, A.E., Elimelech, M. Forward osmosis: Principles, applications, and recent developments. Journal of Membrane Science. 2006. 281: 70-87.
"Summary of the current state of forward osmosis technology."
13. Greenlee, L.F., Lawler, D.F., Freeman, B.D., Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Research. 2009. 43: 2317-2348.
"Summary of the current state of reverse osmosis technology."
14. Maximum Contaminant Levels and Action Levels. Oregon Health Authority website. http://public.health.oregon.gov/HealthyEnvironments/DrinkingWater/Rules/Documents/61-0030.pdf. Accessed January 28, 2016.
"Oregon drinking water maximum contamination limits."
15. Winters, H. Twenty years experience in sea water reverse osmosis and how chemicals in pretreatment affect fouling of membranes. Desalination. 1997. 110: 93-96.
"Fouling of membranes in reverse osmosis."
16. Refrigeration Cycles. Oklahoma University. http://www.ou.edu/class/che-design/che5480-07/Refrigeration%20Basics%20and%20LNG.pdf. Accessed January 27, 2016.
Details on selection of appropriate refrigerant.
17. Williamson, William R, inventor; American Mach & Foundry, assignee. Multistage flash distillation apparatus. U.S. patent 3,399,118. August 27, 1968.
"Basis for our design. MSF with connected chambers, one eductor, and a complete brine dilution recycle."
18. El-Dessouky, H.T., Ettouney, H.M., Al-Roumi, Y. Multi-stage flash desalination: present and future outlook. Chemical Engineering Journal. 1999, 73: 173-190.
"Summary of MSF processes both traditional, and a new recycle method."
19. Kaghazchi, Tahereh, et al. "A mathematical modeling of two industrial seawater desalination plants in the Persian Gulf region." Desalination 252.1 (2010): 135-142. Accessed February 29, 2016.
"Temperature and pressure profiles through multiple connected flash chambers."
20. Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.
"Price comparison between carbon steel and stainless steel; equipment sizing"
21. Malik, A. U., Al-Fozan, S. A. Corrosion and materials selection in MSF desalination plants. Corrosion Reviews. 2011: 29: 153-175.
"Material performance for MSF in the presence of saltwater"
22. Ghani, S., Al-Deffeeri, N. S. Impacts of different antiscalant dosing rates and their thermal performances in Multi Stage Flash (MSF) distiller in Kuwait. Desalination. 2010: 250: 463-472.
"Background information on scaling"
23. Scaling and Antiscalants. Lenntech Water Treatment Solutions website. http://www.lenntech.com/antiscalants.htm. Accessed February 28, 2016.
"Scalant information and pricing"
24. Utility Bill Calculator. City of Newport, Oregon website. http://www.thecityofnewport.net/dept/pwk/billcalc.asp. Accessed February 25, 2016.
"Price of water in Newport, Oregon"
25. Statewide Water Needs Assessment Oregon Water Supply and Conservation Initiative. Oregon Water Resources Department. http://www.oregon.gov/owrd/law/docs/owsci/owrd_demand_assessment_report_final_september_2008.pdf. Accessed January 14, 2016.
"Optimization study about increasing water supply and demand chain problems."