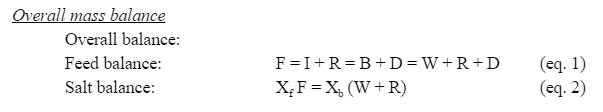Desalination - Team A: Difference between revisions
No edit summary |
|||
| (51 intermediate revisions by 4 users not shown) | |||
| Line 4: | Line 4: | ||
Winter 2016 | Winter 2016 | ||
=Executive Summary= | |||
Seawater desalination is an attractive approach to address the world’s freshwater shortage in light of the increasing global water scarcity. Our team decided to tackle California's growing drought crisis by designing a multistage flash desalination plant in Richmond, CA. Based on market analysis of existing desalination plants and water demands, this is a strategic location for the desalination of 50 million gallons per day of water from the San Francisco Bay. | |||
The MSF process contains 18 flash stages in series. Seawater is taken in from the San Francisco Bay at a rate of 398 million gallons per day and mixed with brine recycled from the system. Within the MSF process, seawater is heated through a series of 18 heat exchangers and a brine heater before enters a series of 18 evaporation chambers. In the evaporation chambers, the seawater flashes and the freshwater vapor is condensed, collected, and sent to a water treatment facility. The brine stream proceeds to the next chamber, and the process continues. Upon exiting the last flashing chamber, the brine stream is split into a recycle brine stream that is mixed with the seawater inlet feed and a waste stream that is diluted with fresh seawater and returned to the sea. Overall, the process yield is 13%. This process was constructed in Aspen HYSYS and optimized around utility costs using the HYSYS Economic Analyzer. Based on duties, temperatures and pressures reported by HYSYS, as well as fundamental mass and energy balances, the equipment were sized appropriately and the economics of the process was analyzed. | |||
The design has a total capital cost of $485.2 MM, an annual operating cost of $144.7 MM ($103.7 MM of which is utilities), and an annual plant revenue of $134.3 MM. Economic analysis reveals that the project has a negative NPV of -$495.7 MM and operates at a loss of $10.4 MM per year. Thus, the proposed design is not profitable. Despite the current projections, it is important to consider the greater implications of running a desalination plant in the Bay Area. The population in the Bay Area is quickly growing while California is experiencing harsher droughts each year. Given the dire situations, the government may support the proposed clean water project with subsidies. Building the facility may be a strategic investment, especially since greater water scarcity is predicted in the future. | |||
Our design meets various environmental, ethical, social and health related constraints. The brine discharged back to the bay is diluted to at most 0.2% above the natural background salinity as required by law so that the plant does not impose harm on any local marine life. The demographic that the plant serves is largely invested in the protection of the environment, which we addressed by reducing energy consumption through direct energy integration with the nearby Chevron plant. Lastly, the product meets the specifications of the California Safe Water Drinking Act by producing water with no impurities. | |||
__TOC__ | |||
=Introduction= | =Introduction= | ||
An increasing global water scarcity is fueling initiatives everywhere for clean water treatment, making efficient seawater desalination an attractive aim for chemical plant design. A 2015 market analysis found that the global desalination market earned revenues of $11.66 billion, and this number is expected to reach over $19 billion by 2019. Furthermore, the 17,000 desalination plants currently in operation are expected to double in number by 2020.<sup>1</sup> | An increasing global water scarcity is fueling initiatives everywhere for clean water treatment, making efficient seawater desalination an attractive aim for chemical plant design. A 2015 market analysis found that the global desalination market earned revenues of $11.66 billion, and this number is expected to reach over $19 billion by 2019. Furthermore, the 17,000 desalination plants currently in operation are expected to double in number by 2020.<sup>1</sup> | ||
As fresh water sources become increasingly scarce, strict water conservation measures are being observed. California is entering the fourth year of one of its most severe droughts on record. Cities are required to reduce their water usage by 35% to avoid facing fines.<sup>2</sup> In lieu of these pressing conditions, California is looking for ways to provide more accessible fresh water to its citizens. This high demand for clean water motivated our choosing Richmond, CA as our plant location. At Richmond, the desalination plant can convert readily available seawater from the San Francisco Bay into fresh water. We propose to desalinate 50 million gallons of water per day to be sent to water treatment plants for potability. This aim is based on the capacity of the desalination plant in Carlsbad, California, which services a similar population. | As fresh water sources become increasingly scarce, strict water conservation measures are being observed. California is entering the fourth year of one of its most severe droughts on record. Cities are required to reduce their water usage by 35% to avoid facing fines.<sup>2</sup> In lieu of these pressing conditions, California is looking for ways to provide more accessible fresh water to its citizens. This high demand for clean water motivated our choosing Richmond, CA as our plant location. At Richmond, the desalination plant can convert readily available seawater from the San Francisco Bay into fresh water. We propose to desalinate 50 million gallons of water per day to be sent to water treatment plants for potability. This aim is based on the capacity of the desalination plant in Carlsbad, California, which services a similar population. | ||
The salinity of the water in the San Francisco | The salinity of the water in the San Francisco Bay is seasonal. During the dry seasons of summer and fall, salinity is high around 10 PSU (10,000 ppm) because water from the Pacific Ocean flows into the San Francisco Bay. During the wet winter season, fresh water from the rivers flows into the Bay, and salinity drops to around 2 PSU (2,000).3 Designed to meet the “worst case” scenario, our plant will process 398 million gallons of water per day with 10,000 ppm of salt to produce 50 million gallons of distilled water. This gives a yield of 13%. | ||
The desalination plant proposed consists of 18 flash stages in series. This report outlines our investigation of this potential water desalination plant, including design and optimization of the desalination process, an analysis of its energy consumption and environmental impacts, and a study of its economic implications. | The desalination plant proposed consists of 18 flash stages in series. This report outlines our investigation of this potential water desalination plant, including design and optimization of the desalination process, an analysis of its energy consumption and environmental impacts, and a study of its economic implications. | ||
| Line 29: | Line 41: | ||
==Pretreatment== | ==Pretreatment== | ||
Sediment is removed to prevent solids from plugging the process downstream. Scaling of the process units leads to decreased efficiency and more frequent unplanned downtime, and is controlled for by lowering pH. Acidifying the water will also remove CO2, which is a corrosive gas. To control for oxygen, oxygen scavengers will be included in the system to sequester it. Sodium bisulfate will be used as the scavenger.<sup>5</sup> | Sediment is removed to prevent solids from plugging the process downstream. Scaling of the process units leads to decreased efficiency and more frequent unplanned downtime, and is controlled for by lowering pH. Acidifying the water will also remove CO2, which is a corrosive gas. To control for oxygen, oxygen scavengers will be included in the system to sequester it. Sodium bisulfate will be used as the scavenger.<sup>5</sup> | ||
==Condensers (Heat Exchanger Networks)== | |||
In order to conserve and recycle energy, the condensation of the distillate and heating of the seawater feed will be coupled in countercurrent heat exchanger networks. These heat exchanger networks are condensers of the design. The cold feed, which is a mixture of fresh seawater and brine recycle, is fed through multiple heat exchangers in series. Each heat exchanger corresponds to a different evaporation chamber. This process consists of 18 heat exchangers: E-101, E-102, … E-117, and E-118, which correspond to evaporation chambers V-102, V-103, … V-118, and V-119, respectively. | |||
The heat exchangers can be viewed similarly to a shell and tube setup, however there is no actual physical shell. The tubes simply run through the top of the evaporation chambers, where vaporized distilled water in the chamber acts as the shell side fluid. The tube bundles are arranged in long tube configuration and are aligned parallel to the direction of the flashing brine flow in the evaporation chamber. The evaporating brine in an evaporation chamber rise up to the the corresponding heat exchanger and condense on the outside of the tubes, creating collectable pure water. This condensing water is what heats up the seawater in the tubes. | |||
E-101 was used as the basis for the condenser sizing since it has the highest heat requirement, and thus will be the largest heat exchanger. From the HYSYS model, the overall system requires 2.24 x 10<sup>6</sup> kW of duty. Assuming this energy requirement, 5000 tubes, and 18 m length tubes (the diameter of the evaporation chambers, explained further on the next page), the diameter of each tube was calculated to be 0.4 m. Based on the temperature of the streams and flow, the thickness of the tube is 7 x 10<sup>-4</sup> m. | |||
==Brine Heater== | |||
After the seawater exits the final heat exchanger, it enters a brine heater (E-119) where it is brought up to the temperature necessary for the flashing process to begin. This means that the seawater must be overheated compared to stage one, so that the seawater will flash in the evaporation chamber, i.e. release heat and vapor to reach equilibrium with stage conditions. After flowing through the condensers, the seawater enters the brine heater at 91.6ºC and is heated to 120ºC. The overall system requires 2.31 x 106 kW of duty. This is supplied by a total of 4.15 x 106 kg per hour of steam. | |||
==Evaporation Chambers== | |||
When the seawater has been brought to a temperature appropriate for flashing, it enters the first evaporation chamber (V-102). Upon entering the chamber, the water is flashed. Some of the water evaporates, leaving the salt behind. The vapor rises up to the heat exchanger tubes in each evaporation chamber and condenses on the outside into the distilled water product. This condensing water is what heats up the seawater in the tubes discussed above. The seawater that does not evaporate moves to the next stage, which operates at a higher temperature and lower pressure, and the process is repeated. This continues in series through a total of 18 flashing chambers (V-102, V-103, … V-118, and V-119). All of the distillate is collected into one stream and all of the brine is mixed with seawater before being disposed of back in the bay in order to dilute it enough so it does not have an adverse effect on marine life. | |||
Each respective heat exchanger fully condenses the vapor product stream from each stage. However, when all of the individual product streams are consolidated into the final combined product stream, some water exists in the vapor phase due to temperature and pressure changes associated with mixing all of the streams together. Although the vapor product stream from each stage condenses completely across its respective heat exchanger, the total product stream contains some water in the vapor phase due to the temperature change when the mixer sums all the streams. In order to fully condense this stream, which will be sent to local water treatment plans, a heat exchanger (E-120) is put in place to cool down the product using the process feed stream. | |||
The correlations of El-Dessouky and Ettouney were employed in order to size the flash chambers. They specified that conventional MSF plants that operate at a capacity between 27,000 and 36,000 m3/day use flashing stages of the dimensions 18 m diameter and 4 m height.<sup>6</sup> | |||
==Valves== | |||
The model requires valves in between the evaporation chambers. The seawater streams flow through valves with large pressure drops to flash a portion the liquid into vapor that can then be separated in the vessels. Large pressure drops provide the thermodynamic driving force to vaporize more of the liquid, and are required to achieve high product yield. | |||
==Material of Construction== | |||
Before 1980, the shells and internals of the MSF units were commonly constructed from carbon steel. Seawater, however, corrodes carbon steel. To compensate for this, the thickness of the carbon steel has an additional corrosion allowance, which increases the size and weight of the equipment. After 1980, the stainless steel and duplex stainless steel became increasingly common. Stainless steel metal requires less thickness for equivalent strength and corrosion resistance, which allows for a reduction in the size, weight, and therefore cost of units. Major units in our process will be constructed from stainless steel.<sup>6</sup> | |||
==Process Alternatives== | |||
In order to ensure that our design is fully optimized, we wanted to consider several alternatives for our process. We looked at alternatives associated with water pretreatment, the distillation process itself and product treatment. | |||
===Water Pretreatment=== | |||
Scaling of the process units leads to decreased efficiency and more frequent unplanned downtime. Scaling can be controlled by adjusting pH, temperature, and bicarbonate concentration. We selected acidification to control for process scaling. This is because lowering a process temperature would decrease overall efficiency, and decreasing bicarbonate concentration would be comparatively expensive. Lowering pH would have the added benefit of controlling CO2 concentrations since CO2 is a corrosive gas. To control for corrosion we looked at how to remove oxygen. To target the oxygen we chose an oxygen scavenger. This was chosen over other options such as a deaerator, or a protective coating because a deaerator would have a large capital cost, and the equipment itself would experience corrosion, and a tank coating such as zinc orthophosphate has a mixed effectiveness.<sup>7</sup> | |||
===MSF: Brine Recycle vs. Once-Through System=== | |||
We explored two options for the overall layout of the MSF distillation plant. In the first, the brine passes through the process once; in the second, a portion of the brine is recycled from the last stage to the incoming seawater feed. The largest draw towards implementing a brine recycle is that it would significantly reduce the overall amount of seawater needed to produce the daily target of 50 million gallons per day. This in turn would lower the amount of energy required to run the pump feeding seawater to the process. Another positive is that, with a brine recycle, the amount of water conditioning chemicals that must be added to the seawater feed is reduced. The negative aspects mainly revolve around the fact that the solution throughout the process will be higher in salt concentration. This increase in salinity both raises the likelihood of scaling and corrosion in the plant and raises the amount of seawater that must be added to the final brine to make it safe for discharge back into the environment. Additionally, boiling point of each stage is raised when adding the brine recycle. After weighing both alternatives against one another, we have decided to include a brine recycle in our plant design. Most of the negative aspects of the brine recycle can be combated (e.g. include anti-scaling agents in the feed pretreatment), and the economic benefits of including the brine recycle are very significant.<sup>8</sup> | |||
===MSF: Continuous vs. Batch=== | |||
The distillation step can be done in continuous or batch processes. Batch distillation is more versatile and often used when the product is in small amounts and very high purity. A continuous distillation is more efficient thermodynamically and economically for large amounts of material of constant composition, whereas batch distillation is more effective for small amounts of material of varying compositions. Since the plant is processing large amounts of seawater of relatively constant composition, the distillation system will utilize continuous distillation.<sup>9</sup> | |||
===MSF: Long Tube vs. Crossed Tube === | |||
Inside the evaporator stage, the condenser tube bundles may be arranged in either long tube or cross tube design. In the long tube arrangement, the tubes are aligned parallel to the direction of the flashing brine flow. The tubes go from stage to stage without water boxes. In the cross tube arrangement, the tubes are lined perpendicular to the flow of the flashing brine. Water boxes transfer brine between the stages.<sup>10 11</sup> While the water boxes in crossed tube bundles allow for more customization, the long tube configuration allows for construction of large unit sizes, as there are manufacturing restrictions with cross tubes. Long tube design can easily accommodate a large number of stages, which lead us to choose long tube for our design.<sup>11 12</sup> | |||
===MSF: Venting System=== | |||
Noncondensable gases exist in the process because of air leakages into stages under vacuum conditions and the release of dissolved gases from the heated brine. These gases are removed by venting the system. An air cooler section concentrates the gas before they are vented, and baffles direct the vapor and condensates out. There are two venting arrangements to consider: series and parallel. The system in series moves gas from one stage directly to the next, and the only loss in the system is vapor in the last stage (since some vapor is extracted with gases). This system is economical, however this configuration increases the amount of noncondensable gases passing from one stage to the next, and disrupting the venting system in one stage will affect the entire system. In parallel venting, gas is released through parallel takeoff pipes at each stage. Although this arrangement allows for venting at any stage to be controlled, the design and maintenance is more complex, and more vapor is lost than in the venting system in series. A third alternative is to use a combination of venting in parallel and in series, which is the common method in industry. For this process, our team chose to do venting in parallel and in series.<sup>12</sup> | |||
===Additional Energy Source Alternatives=== | |||
Energy for the process can also be coupled with renewable energy sources to reduce the desalination plant’s carbon footprint. Energy integration can be direct or indirect. In direct integration, thermal waste heat from industrial sources provide thermal energy or pressure as energy sources. Direct integration is advantageous at the modular level, as it avoids energy losses with electricity conversion. Within indirect integration, there are modular integration and grid/utility-scale integration. Modular integration integrates the plant with wind turbines and other small-scale renewable power generators with mobile deployment. A “hybridization” of renewable power sources with natural gas power generation is also an option, and the coupling of the two provides a stable power source. Indirect renewable energy integration is growing as the cost of renewable energy continues to decreases and provides an economy of scale that direct integration does not, but there are several obstacles towards indirect integration, such as the plant’s finance and engineering the integration of the two systems.<sup>13</sup> This distillation plant will be directly integrated with neighboring plants such as Chevron that produce thermal waste heat. | |||
===Product Treatment=== | |||
Since the product is distilled water, it needs additional ions to a concentration of around 0.01% before going to the end user. The three alternatives considered were building a water treatment facility on site, simply adding the correct salts to the product stream and then sending the stream for bacterial treatment at a water plant, or simply sending the product distilled water for complete treatment to another facility. Due to the number of nearby water treatment plants and their demand for water, we elected to simply send the product to a nearby water treatment facility. | |||
==Design Constraints and Tradeoffs== | |||
While designing our process, we wanted to make sure that we met several outside constraints, including environmental, political, ethical, social, sustainability, health and safety constraints. First and most importantly, we wanted to ensure that the brine discharged back to the bay would not harm local marine life. Our process was designed around the constraint of diluting the exiting brine to within 0.2% salinity of background seawater. This is not only compliant with California law, but was also verified to be safe for surrounding marine life through our own outside research. We also addressed many of these considerations by deciding to pursue direct energy integration with the nearby Chevron plant in order to reduce environmental impact of our process. Additionally, we ensured that our plant would be located in an area that is densely populated and in great need of fresh drinking water. Finally, we addressed health and safety considerations by making sure we would be able to send our product distilled water to local treatment plants for proper potabilization. | |||
===Design Tradeoffs: Thermodynamics=== | |||
Adjusting elements within this process is iterative, and optimization thereof required changing multiple parameters at once. An example of this is in tuning the temperature increase by heater E-100; if the temperature of the stream entering the vessels is too high, the heat exchange between the vapor product and the feed stream will not be realizable because it causes the feed to be too hot to cool and condense the product. Higher temperatures, however, provide greater thermodynamic driving forces for flashing. Setting the entering stream temperature at 120°C provides enough heat for substantial flash at 130 kPa. This is the only heater required until the very last flash stage, which is added to provide more heat for vaporization. This heater (E-105) increases the stream temperature by 15°C, and the vapor product from the last vessel increases the feed stream temperature by almost 30°C. The added energy increases vaporization by 3%, which is a substantial 2.2 million kg/h. The temperature of the streams entering and leaving each stage decreases with each additional flash stage such that the last vessel has the lowest temperature, according to realistic operating guidelines. | |||
===Design Tradeoffs: Energy Consumption, Environmental Impacts, and Product Optimization=== | |||
Increases in the pressure drop across any of the valves in this final design causes a temperature cross in the heat exchangers unless a proper increase in pump energy is provided. There is thus a design tradeoff between lowering energy usage and increasing product yield. For example, lowering the operating pressure of the process dictates that the pressure difference used to flash seawater must be smaller, possibly decreasing the amount that is vaporized. The final design aims for a yield of 50 million gallons per day. The 18 stage model was chosen for its low annual utility cost. This is a reflection of environmental mindfulness because energy consumption is an ongoing aspect of the process and should be lowered as much as possible. | |||
==Mass and Energy Balances== | |||
The model of the desalination system assumes that: (1) the properties are uniform in each phase within a stage, (2) heat losses are negligible due to the small surface area to volume ratio for each stage and properly insulated units (heat losses to the surrounding vary from 2 to 5% of the total system energy), (3) the distillate is salt free since the boiling point of water is much lower than that of salt, (4) the subcooling or superheating effects on the system energy balances are negligible, (5) the only contaminant is sodium chloride (there are no non-condensable gases to consider, so no vapors are vented), and (6) the process has 3 evaporation chambers. | |||
The major balances are that the feed is a mixture of the fresh seawater stream and the recycle stream, and that the brine stream exiting the last evaporation chamber is divided into the recycle stream and the waste stream. Within each stage, the change in mass of a phase over time is the balance between the net flow of that phase from the chamber and the rates of evaporation and condensation of water into and out of that phase. The equations governing the mass and energy balances on the system is displayed in Appendix C. | |||
=Optimization= | |||
As initiatives for reduced energy usage and increased environmental sustainability become more prevalent, efforts to minimize the utility cost in plants has increased to accommodate these incentives. Utilities are a continual source of expenditure and consumption, and a well-designed chemical process should seek to minimize this element. The annualized utility cost is a useful parameter to optimize given that its value depends on variations in energy costs; thus, annualized utility costs do not correlate with conventional indices of inflation, such as capital and labor costs. Additionally, utility costs, like other variable costs, can be minimized by improving the design or operational efficiency of the plant. | |||
In optimizing our MSF process, the design variable of focus was the number of stages to use in our process. The performance measure was the annualized utility cost, which was the parameter that was minimized for optimization. The process is constrained by the product specifications, which are kept constant and serve as the standard for comparison between each model. These specifications are to produce 50 million gallons of desalinated liquid water per day. | |||
One feasibility constraint in the process is the size of the heat exchangers, which must provide a large enough area for total condensation of the vapor stream coming out of the flash stages. In order to restrict the heat exchange area needed within each exchanger, the number of stages must be at least 11. Increasing the number of stages decreases the vapor flow rate at each, and decreases the heat exchange area required as well. An 11-stage process allows for appropriate heat exchange between the feed stream and the vapor product, according to the HYSYS economic analyzer. The optimization analysis thus was conducted incorporating only models with 11 or more stages. | |||
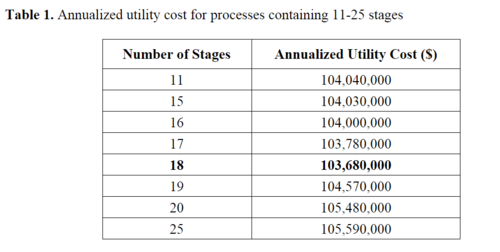
The HYSYS economic analyzer was used to systematically optimize the process by finding the model with the lowest annualized utility cost. The performance parameters kept constant across each model were: the inlet composition, pressure, and temperature, the brine outlet temperature, the total product flow rate and composition, and the flash stage schematic. Table 1 displays the annualized utility cost for each model, and illustrates that a clear dip in the utility cost occurs in an 18 stage model. Thus, 18 is the optimal number of stages to use for our MSF distillation given utility costs as the optimized parameter. | |||
[[File:Table_1.PNG|center|500x300px]] | |||
=Economic Evaluation= | |||
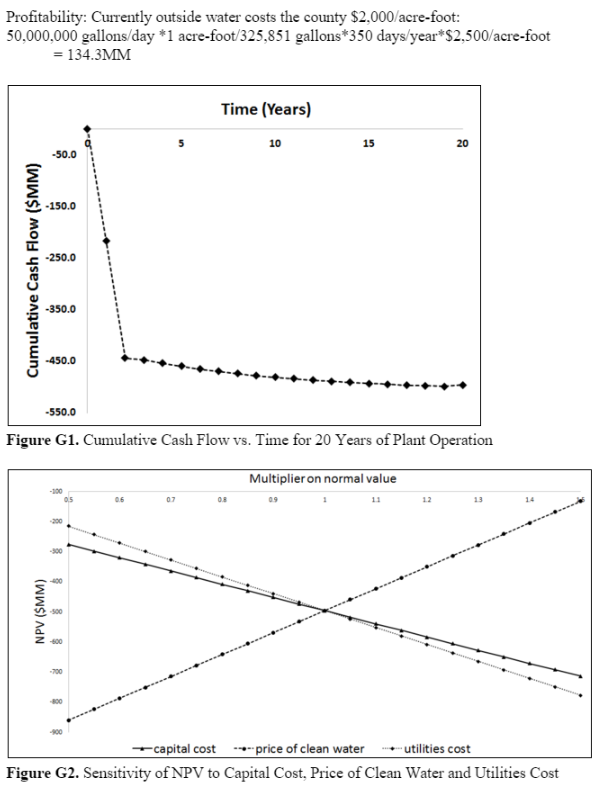
In order to evaluate our process from a fiscal standpoint, we used Aspen Process Economic Analyzer and Aspen cost estimator to determine capital and operating costs for our plant. These costs were calculated based on calculated equipment sizes, as well as energy requirements reported by our HYSYS model. The capital cost was found to be $485.2MM and annual total operating cost was found to be $144.7MM ($103.7MM of which was utilities). Annual plant revenue was estimated based on prices that water districts in the county currently pay for outside water sources, and was found to be $134.3MM.<sup>14</sup> | |||
The NPV analysis we conducted assumed that the plant would take two years to build, would operate at half capacity during the first year of operation (year 3), and have a 20 year lifetime. We also assumed that our plant would be in operation 350 days per year. A constant discount rate of 12% was assumed to hold throughout the plant’s lifetime. The final NPV of this project is -$495.7MM. This is due to both the large capital cost of the plant and the annual loss of $10.4MM while in full operation. A graph of cumulative cash flow versus time can be found in Appendix E. Tax rates and depreciation do not directly impact the NPV of this project due to operating at a loss, however for future calculations it should be noted that we used a straight line depreciation schedule over a seven year period with a 40% tax rate. | |||
Despite the current projections, it is important to consider the greater implications of running a desalination plant in the Bay Area. The population in the Bay Area is quickly growing, while California is experiencing harsher droughts each year. A consequence of this is the possibility of receiving government subsidies for our clean water product. The similarly scaled desalination plant in Carlsbad, CA operates at a loss as well, but receives significant government subsidies. Building this facility may be a strategic investment, especially since greater water scarcity is predicted in the future. | |||
=Sensitivity Analysis= | |||
We wanted to determine how sensitive NPV of our project is to the cost of capital, price of utilities and price we would be able to sell our clean water product at. We conducted this analysis by varying each of these variables independently between 0.5 times and 1.5 times the original value we calculated. As to be expected, NPV grows more negative with increasing capital costs and utility prices and decreasing water prices. Conversely, NPV becomes less negative as price of water rises and capital and utility costs decrease. A plot summarizing the effects of varying these costs can be found in Appendix E. NPV is most sensitive to the price at which we will be selling our clean water product. It is worth noting that NPV is more sensitive to utilities cost than it is to capital cost, which is why we focused on optimizing the cost of utilities. In future steps, trying to reduce utilities cost even more would be worth looking into to decrease the amount of money being lost on this project. | |||
=Conclusions and Final Recommendation= | |||
The final design achieves the project goal of producing 50 million gallons per day of distilled water at a yield of 13%, comparable to other similarly sized plants. An optimization of the number of MSF stages is realistic, and can be improved upon by taking into account other process parameters. | |||
The proposed design was optimized around utility costs, comparing plants with 11 to 25 stages. The process is constrained by product specifications and by the thermodynamics of the heat exchange between the feed and the product streams. Energy conservation was achieved in using the seawater feed to condense the final product, as well as in all of the heat exchangers increasing the thermal energy of the seawater feed stream before it enters the flash stages. | |||
In order to further optimize this process, other design parameters should be considered such as the brine recycle ratio, the cooler duty of the recycle stream, the pressure drop across each valve at the flash stages, and other economic factors. An optimization around maximizing NPV, for example, might be more comprehensive than that conducted regarding only utility costs. Tuning brine recycle rates could greatly improve process efficiency, and standardizing flash stage pressure drops would make operations more feasible and controlled. | |||
Given our in depth economic analysis, this project would lose an average of $10.4MM per year, and has an NPV of -$495.7MM for a 20 year plant lifetime. From an economic perspective alone it would not be a good project to build. However, other factors need to be considered when building a project with enormous public value. In recent years California droughts have become more severe. Implementing a drought-proof supply of water now could avert a disaster later on. Even without a large disaster, having the desalination plant could also be expected to pay off in the future with the increasing price of water. Considerations such as these were the deciding factors in building the Carlsbad desalination plant. Since there are currently no desalination plants in the Bay area we recommend that preparations should be made to move forward with a desalination plant. | |||
=References= | |||
[1] Lyons J. Environmental Leader. Environmental Leader 2015. Available at: http://www.environmentalleader.com/2015/10/27/desalination-plants-to-double-by-2020. Accessed 2016. | |||
[2] Fitzsimmons E. In California, Cities Braced to Cut Water by 10 to 35%. The New York Times 2015. Available at: | |||
http://www.nytimes.com/2015/04/09/us/in-california-cities-braced-to-cut-water-by-10-to-35.html. Accessed 2016. | |||
[3] Salinity Times Series - USGS Water Quality of SF Bay. Salinity Times Series - USGS Water Quality of SF Bay. Available at: | |||
http://sfbay.wr.usgs.gov/access/wqdata/overview/examp/charts/salin.html. Accessed 2016. | |||
[4] Reverse Osmosis vs. Multi Stage Flash Distillation: A Comparison Between Different Desalination Methods. Brighthub Engineering. Available at: | |||
http://www.brighthubengineering.com/power-plants/29621-comparison-between-the-reverse-osmosis-and-multi-stage-flash-distillation-methods/. Accessed 2016. | |||
[5] Committee on Advancing Desalination Technology, Water Science and Technology Board, Division on Earth and Life Studies, National Research Council. Desalination: A National Perspective. Washington, D.C.:The National Academies Press, 2008. | |||
[6] H. El-Dessouky. Fundamentals of salt water desalination, 1st Ed., Elsevier Science, 2002. | |||
[7] Committee on Advancing Desalination Technology, Water Science and Technology Board, Division on Earth and Life Studies, National Research Council. Desalination: A National Perspective. Washington, D.C.:The National Academies Press, 2008. | |||
[8] Guasp RE, Bello CP, Jara JG, Vega JP, Damien T, Winkel C, Smith HH. Desalination by distillation. Organization of American States website. oas.org. Accessed January 28, 2015. | |||
[9] Batch Distillation. AE 335 Separation Processes website. http://prodpran.che.engr.tu.ac.th/AE335/AE335.html. Accessed January 26, 2016. | |||
[10] Morris R. The development of the multi-stage flash distillation process: A designer's viewpoint. Desalination. 1993;93:57-68. | |||
[11] Darwish MA. Developments in the multi-stage flash desalting system. Desalination. 1995;100:35-64. | |||
[12] Wade N. Distillation plant development and cost update. Desalination. 2001;136:3-12. | |||
[13] Desalination Technologies and the Use of Alternative Energies for Desalination. World Intellectual Property Organization website. http://www.wipo.int/export/sites/www/patentscope/ | |||
en/programs/patent_landscapes/documents/patent_landscapes/948-2E-WEB.pdf. Updated November 2011. Accessed January 23, 2016. | |||
[14] California Turns to the Pacific Ocean for Water | MIT Technology Review.MIT Technology Review 2014. Available at: | |||
http://www.technologyreview.com/featuredstory/533446/desalination-out-of-desperation/. Accessed 2016. | |||
---- | |||
---- | |||
---- | |||
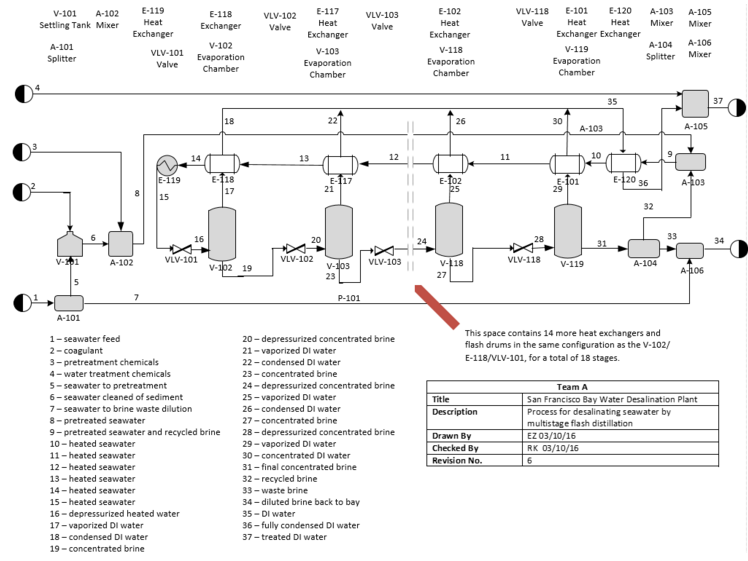
=Appendix A. Process Flow Diagram= | |||
[[File:Appendix 1.PNG]] | |||
'''Figure A1''' Process Flow Diagram of the MSF System | |||
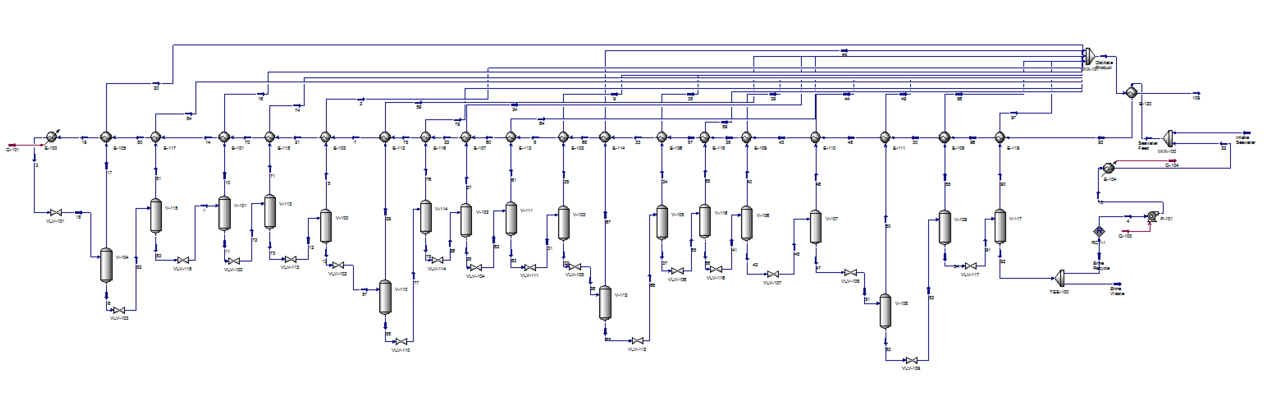
=Appendix B. HYSYS Simulation= | |||
The HYSYS model of the process is shown below. | |||
[[File:AppendB.PNG]] | |||
'''Figure B1.''' The HYSYS model of the MSF distillation process. | |||
=Appendix C. Mass and Energy Balances= | |||
Below are equations that describe the mass and energy balances of the system. | |||
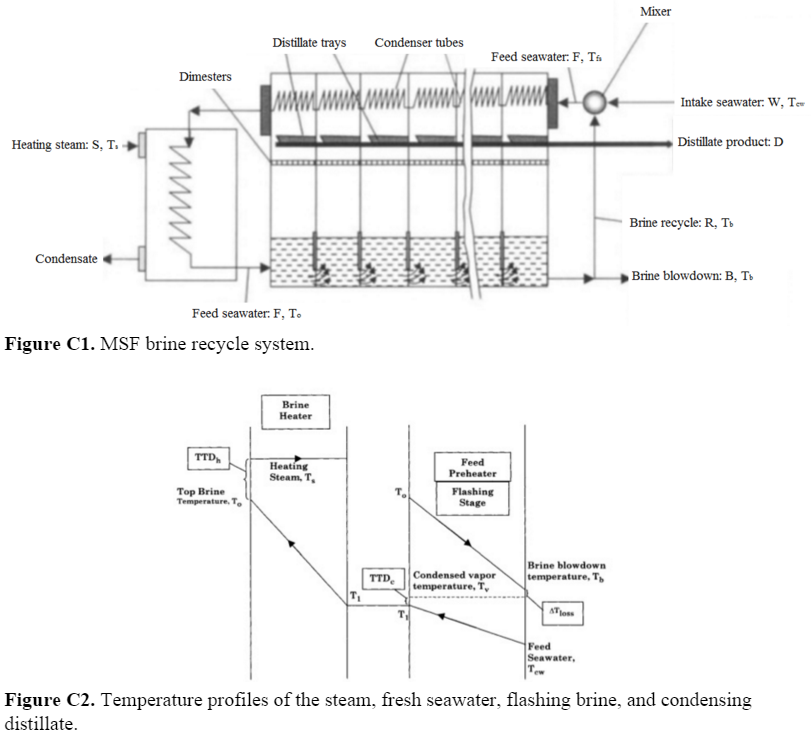
In MSF system with brine recycle, the mass flow of the intake seawater I is mixed with the mass flow of the recycle brine R to create the feed seawater stream F. Since this model assumes no non-condensable gases, the feed stream is overall balanced by the brine waste stream W, the recycle brine stream, and the distillate stream D. The brine stream exiting the last evaporation chamber B is divided into the recycle and waste streams. The amount of salt that enters the series of evaporation chambers is the amount of salt that leaves (X is the salt mass fraction). | |||
[[File:Overallmasss.PNG]] | |||
Within each stage, the change in mass of a phase is the mass flows of that phase into and out of the chamber and the rates of evaporation or condensation of the water into and out of that phase. | |||
[[File:Masss2.PNG]] | |||
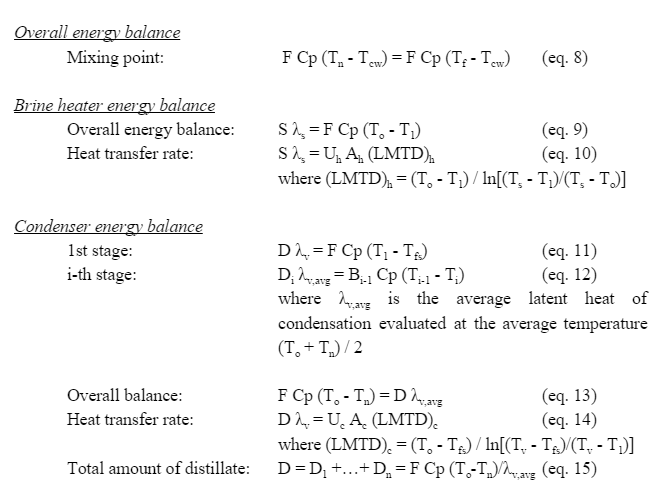
The energy balances are summarized below. | |||
[[File:Mass3.PNG]] | |||
[[File:Mass4.PNG]] | |||
=Appendix D. Summary of Process Units= | |||
[[File:AppendD1.PNG]] | |||
[[File:AppenD2.PNG]] | |||
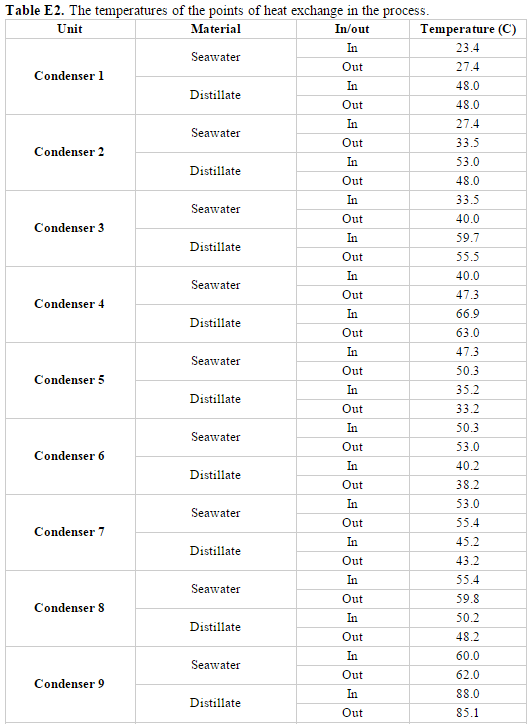
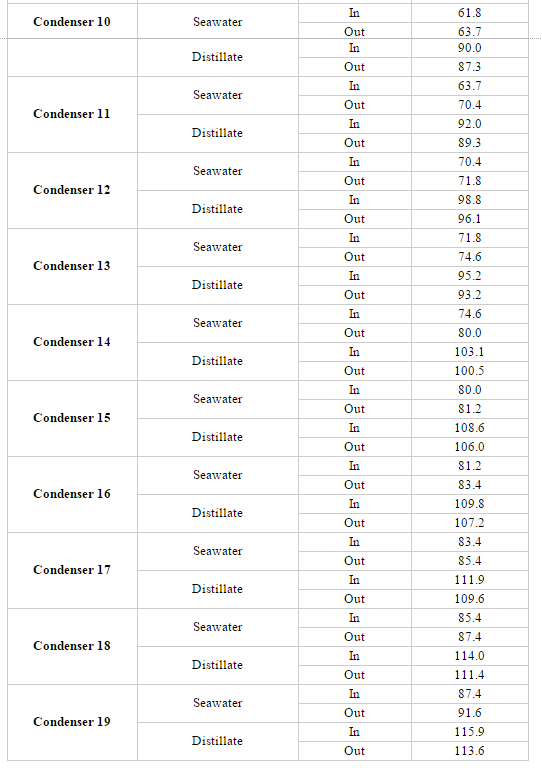
=Appendix E. MSF Stage Parameters= | |||
[[File:AppendE.PNG]] | |||
[[File:Appendix E3.PNG]] | |||
[[File:Appendix E4.PNG]] | |||
=Appendix F. Process Units Calculations= | |||
[[File:AppendF.PNG]] | |||
[[File:AppendF2.PNG]] | |||
[[File:AppendF3.PNG]] | |||
=Appendix G. Economic Analysis= | |||
[[File:Econanalysis.PNG]] | |||
Latest revision as of 03:34, 12 March 2016
Author: Pear Dhiantravan[2016], Reed Kolbe[2016], Sheridan Lichtor[2016], John Marsiglio[2016], Ellen Zhuang[2016]
Instructors: Fengqi You, David Wegerer
Winter 2016
Executive Summary
Seawater desalination is an attractive approach to address the world’s freshwater shortage in light of the increasing global water scarcity. Our team decided to tackle California's growing drought crisis by designing a multistage flash desalination plant in Richmond, CA. Based on market analysis of existing desalination plants and water demands, this is a strategic location for the desalination of 50 million gallons per day of water from the San Francisco Bay.
The MSF process contains 18 flash stages in series. Seawater is taken in from the San Francisco Bay at a rate of 398 million gallons per day and mixed with brine recycled from the system. Within the MSF process, seawater is heated through a series of 18 heat exchangers and a brine heater before enters a series of 18 evaporation chambers. In the evaporation chambers, the seawater flashes and the freshwater vapor is condensed, collected, and sent to a water treatment facility. The brine stream proceeds to the next chamber, and the process continues. Upon exiting the last flashing chamber, the brine stream is split into a recycle brine stream that is mixed with the seawater inlet feed and a waste stream that is diluted with fresh seawater and returned to the sea. Overall, the process yield is 13%. This process was constructed in Aspen HYSYS and optimized around utility costs using the HYSYS Economic Analyzer. Based on duties, temperatures and pressures reported by HYSYS, as well as fundamental mass and energy balances, the equipment were sized appropriately and the economics of the process was analyzed.
The design has a total capital cost of $485.2 MM, an annual operating cost of $144.7 MM ($103.7 MM of which is utilities), and an annual plant revenue of $134.3 MM. Economic analysis reveals that the project has a negative NPV of -$495.7 MM and operates at a loss of $10.4 MM per year. Thus, the proposed design is not profitable. Despite the current projections, it is important to consider the greater implications of running a desalination plant in the Bay Area. The population in the Bay Area is quickly growing while California is experiencing harsher droughts each year. Given the dire situations, the government may support the proposed clean water project with subsidies. Building the facility may be a strategic investment, especially since greater water scarcity is predicted in the future.
Our design meets various environmental, ethical, social and health related constraints. The brine discharged back to the bay is diluted to at most 0.2% above the natural background salinity as required by law so that the plant does not impose harm on any local marine life. The demographic that the plant serves is largely invested in the protection of the environment, which we addressed by reducing energy consumption through direct energy integration with the nearby Chevron plant. Lastly, the product meets the specifications of the California Safe Water Drinking Act by producing water with no impurities.
Introduction
An increasing global water scarcity is fueling initiatives everywhere for clean water treatment, making efficient seawater desalination an attractive aim for chemical plant design. A 2015 market analysis found that the global desalination market earned revenues of $11.66 billion, and this number is expected to reach over $19 billion by 2019. Furthermore, the 17,000 desalination plants currently in operation are expected to double in number by 2020.1
As fresh water sources become increasingly scarce, strict water conservation measures are being observed. California is entering the fourth year of one of its most severe droughts on record. Cities are required to reduce their water usage by 35% to avoid facing fines.2 In lieu of these pressing conditions, California is looking for ways to provide more accessible fresh water to its citizens. This high demand for clean water motivated our choosing Richmond, CA as our plant location. At Richmond, the desalination plant can convert readily available seawater from the San Francisco Bay into fresh water. We propose to desalinate 50 million gallons of water per day to be sent to water treatment plants for potability. This aim is based on the capacity of the desalination plant in Carlsbad, California, which services a similar population.
The salinity of the water in the San Francisco Bay is seasonal. During the dry seasons of summer and fall, salinity is high around 10 PSU (10,000 ppm) because water from the Pacific Ocean flows into the San Francisco Bay. During the wet winter season, fresh water from the rivers flows into the Bay, and salinity drops to around 2 PSU (2,000).3 Designed to meet the “worst case” scenario, our plant will process 398 million gallons of water per day with 10,000 ppm of salt to produce 50 million gallons of distilled water. This gives a yield of 13%.
The desalination plant proposed consists of 18 flash stages in series. This report outlines our investigation of this potential water desalination plant, including design and optimization of the desalination process, an analysis of its energy consumption and environmental impacts, and a study of its economic implications.
Technical Approach
For the desalination step of our process, we selected a multistage flash (MSF) distillation process. Not only is MSF distillation a very viable method, but it is also the most common method, currently producing about 60% of the world’s desalinated water. MSF and reverse osmosis (RO) are the two major methods being used in large-scale desalination plants. Both processes require considerable amounts of energy. RO typically has a lower energy demand; however, the high impurity content of the Bay water would frequently necessitate membrane cleaning and/or exchange. Feed going through a RO system requires extensive pretreatment to remove biological organisms and other solids to control the pH and the chemical composition of the water. With the San Francisco Bay as our source of water, MSF is an attractive option because sediment and other large impurities can be separated from the feed before distillation occurs. Additionally, MSF distillation plants can be located near power plants and paired to their waste heat streams to conserve energy. This can reduce energy needs by half or two thirds, making MSF increasingly more practical. Due to the maintenance required in the RO system, the MSF distillation is the better option in terms of operation cost.4
A HYSYS model was built to simulate the MSF distillation section of the process, which spans after seawater pretreatment and before local potable water treatment. The model is used to simulate the distillation steps and to calculate necessary heating, cooling, and input flow rates to produce the required 50 million gallons of distillated water per day. For the simulation, the 398 million gallons per day of seawater feed stream is defined to be composed of 3.31 mol% sodium chloride (NaCl) and 96.69 mol% water. The percentage of NaCl is set to account for other ions and chemicals present after the pretreatment phase. NaCl alone is added to the HYSYS system due to the capabilities of the fluid package used to simulate this process, ElectroNRTL. The feed is set at 22°C, which is the approximate the temperature of the San Francisco Bay year round.
Process Design
Design Overview
The proposed desalination plant uses MSF distillation to convert the San Francisco Bay water to fresh water. The final process design contains 18 flash stages in series. Overall, 398 million gallons per day of of brackish water is converted to 50 million gallons per day of deionized water, a 13% yield. The production meets the required production rate of 50 million gallons of freshwater per day.
Seawater is taken in from the San Francisco Bay and mixed with the brine recycle stream produced downstream. The feed is then heated through a series of condensers and a heater. Distilled water vapor in each flash stage contacts the tubes carrying the seawater feed and condenses into a liquid stream by exchanging heat with the cooler seawater feed stream. The seawater is sent through a heater to increase its thermal energy before entering the first stage of the MSF. In each flashing stage, some of the water evaporates, leaving the salt behind. The freshwater vapors condense, as described above, and is collected and sent to a water treatment plant. The brine stream is split into the recycle stream and a waste stream, and the waste stream is diluted with fresh seawater before being discharged back into the bay.
A process flow diagram of the process is depicted in Appendix A.
Pretreatment
Sediment is removed to prevent solids from plugging the process downstream. Scaling of the process units leads to decreased efficiency and more frequent unplanned downtime, and is controlled for by lowering pH. Acidifying the water will also remove CO2, which is a corrosive gas. To control for oxygen, oxygen scavengers will be included in the system to sequester it. Sodium bisulfate will be used as the scavenger.5
Condensers (Heat Exchanger Networks)
In order to conserve and recycle energy, the condensation of the distillate and heating of the seawater feed will be coupled in countercurrent heat exchanger networks. These heat exchanger networks are condensers of the design. The cold feed, which is a mixture of fresh seawater and brine recycle, is fed through multiple heat exchangers in series. Each heat exchanger corresponds to a different evaporation chamber. This process consists of 18 heat exchangers: E-101, E-102, … E-117, and E-118, which correspond to evaporation chambers V-102, V-103, … V-118, and V-119, respectively.
The heat exchangers can be viewed similarly to a shell and tube setup, however there is no actual physical shell. The tubes simply run through the top of the evaporation chambers, where vaporized distilled water in the chamber acts as the shell side fluid. The tube bundles are arranged in long tube configuration and are aligned parallel to the direction of the flashing brine flow in the evaporation chamber. The evaporating brine in an evaporation chamber rise up to the the corresponding heat exchanger and condense on the outside of the tubes, creating collectable pure water. This condensing water is what heats up the seawater in the tubes.
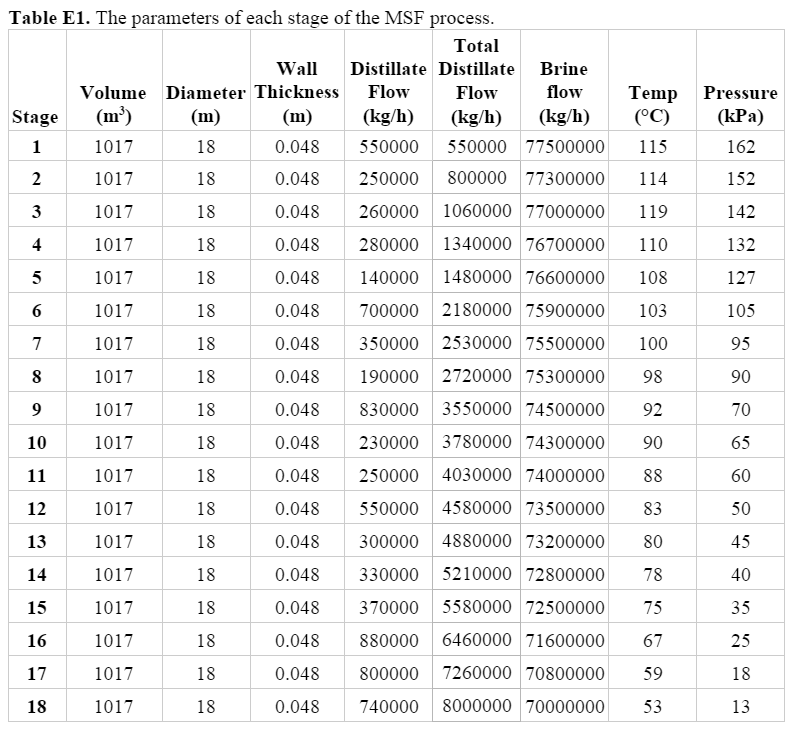
E-101 was used as the basis for the condenser sizing since it has the highest heat requirement, and thus will be the largest heat exchanger. From the HYSYS model, the overall system requires 2.24 x 106 kW of duty. Assuming this energy requirement, 5000 tubes, and 18 m length tubes (the diameter of the evaporation chambers, explained further on the next page), the diameter of each tube was calculated to be 0.4 m. Based on the temperature of the streams and flow, the thickness of the tube is 7 x 10-4 m.
Brine Heater
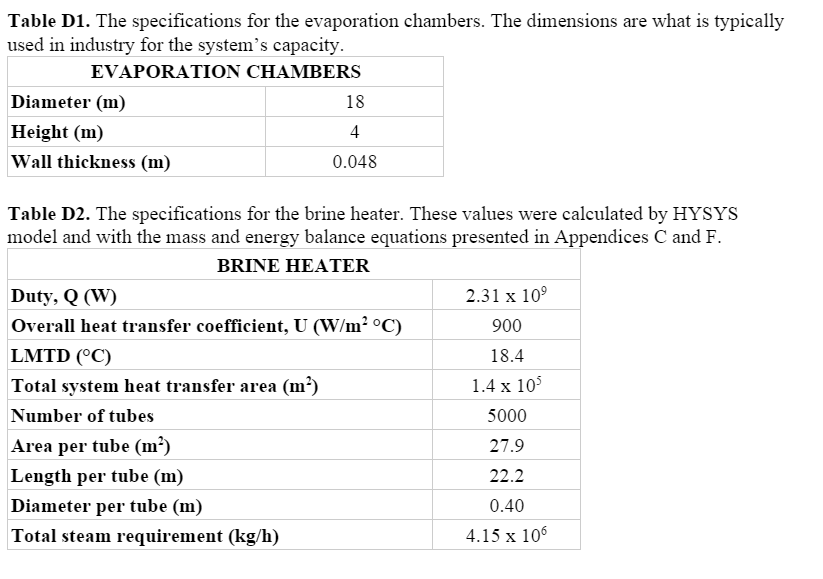
After the seawater exits the final heat exchanger, it enters a brine heater (E-119) where it is brought up to the temperature necessary for the flashing process to begin. This means that the seawater must be overheated compared to stage one, so that the seawater will flash in the evaporation chamber, i.e. release heat and vapor to reach equilibrium with stage conditions. After flowing through the condensers, the seawater enters the brine heater at 91.6ºC and is heated to 120ºC. The overall system requires 2.31 x 106 kW of duty. This is supplied by a total of 4.15 x 106 kg per hour of steam.
Evaporation Chambers
When the seawater has been brought to a temperature appropriate for flashing, it enters the first evaporation chamber (V-102). Upon entering the chamber, the water is flashed. Some of the water evaporates, leaving the salt behind. The vapor rises up to the heat exchanger tubes in each evaporation chamber and condenses on the outside into the distilled water product. This condensing water is what heats up the seawater in the tubes discussed above. The seawater that does not evaporate moves to the next stage, which operates at a higher temperature and lower pressure, and the process is repeated. This continues in series through a total of 18 flashing chambers (V-102, V-103, … V-118, and V-119). All of the distillate is collected into one stream and all of the brine is mixed with seawater before being disposed of back in the bay in order to dilute it enough so it does not have an adverse effect on marine life.
Each respective heat exchanger fully condenses the vapor product stream from each stage. However, when all of the individual product streams are consolidated into the final combined product stream, some water exists in the vapor phase due to temperature and pressure changes associated with mixing all of the streams together. Although the vapor product stream from each stage condenses completely across its respective heat exchanger, the total product stream contains some water in the vapor phase due to the temperature change when the mixer sums all the streams. In order to fully condense this stream, which will be sent to local water treatment plans, a heat exchanger (E-120) is put in place to cool down the product using the process feed stream.
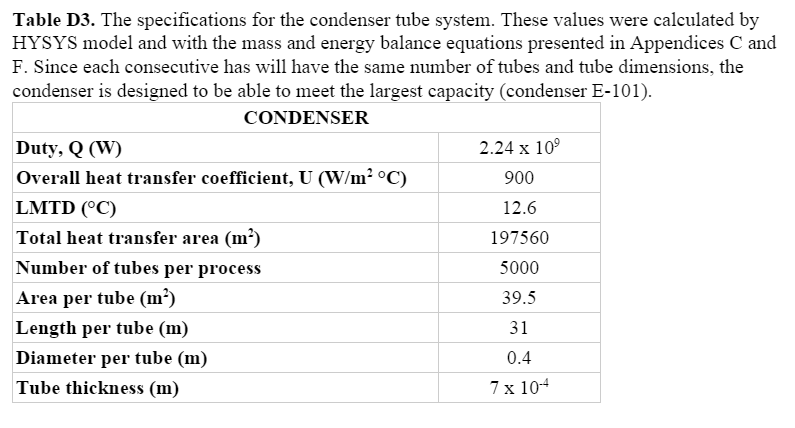
The correlations of El-Dessouky and Ettouney were employed in order to size the flash chambers. They specified that conventional MSF plants that operate at a capacity between 27,000 and 36,000 m3/day use flashing stages of the dimensions 18 m diameter and 4 m height.6
Valves
The model requires valves in between the evaporation chambers. The seawater streams flow through valves with large pressure drops to flash a portion the liquid into vapor that can then be separated in the vessels. Large pressure drops provide the thermodynamic driving force to vaporize more of the liquid, and are required to achieve high product yield.
Material of Construction
Before 1980, the shells and internals of the MSF units were commonly constructed from carbon steel. Seawater, however, corrodes carbon steel. To compensate for this, the thickness of the carbon steel has an additional corrosion allowance, which increases the size and weight of the equipment. After 1980, the stainless steel and duplex stainless steel became increasingly common. Stainless steel metal requires less thickness for equivalent strength and corrosion resistance, which allows for a reduction in the size, weight, and therefore cost of units. Major units in our process will be constructed from stainless steel.6
Process Alternatives
In order to ensure that our design is fully optimized, we wanted to consider several alternatives for our process. We looked at alternatives associated with water pretreatment, the distillation process itself and product treatment.
Water Pretreatment
Scaling of the process units leads to decreased efficiency and more frequent unplanned downtime. Scaling can be controlled by adjusting pH, temperature, and bicarbonate concentration. We selected acidification to control for process scaling. This is because lowering a process temperature would decrease overall efficiency, and decreasing bicarbonate concentration would be comparatively expensive. Lowering pH would have the added benefit of controlling CO2 concentrations since CO2 is a corrosive gas. To control for corrosion we looked at how to remove oxygen. To target the oxygen we chose an oxygen scavenger. This was chosen over other options such as a deaerator, or a protective coating because a deaerator would have a large capital cost, and the equipment itself would experience corrosion, and a tank coating such as zinc orthophosphate has a mixed effectiveness.7
MSF: Brine Recycle vs. Once-Through System
We explored two options for the overall layout of the MSF distillation plant. In the first, the brine passes through the process once; in the second, a portion of the brine is recycled from the last stage to the incoming seawater feed. The largest draw towards implementing a brine recycle is that it would significantly reduce the overall amount of seawater needed to produce the daily target of 50 million gallons per day. This in turn would lower the amount of energy required to run the pump feeding seawater to the process. Another positive is that, with a brine recycle, the amount of water conditioning chemicals that must be added to the seawater feed is reduced. The negative aspects mainly revolve around the fact that the solution throughout the process will be higher in salt concentration. This increase in salinity both raises the likelihood of scaling and corrosion in the plant and raises the amount of seawater that must be added to the final brine to make it safe for discharge back into the environment. Additionally, boiling point of each stage is raised when adding the brine recycle. After weighing both alternatives against one another, we have decided to include a brine recycle in our plant design. Most of the negative aspects of the brine recycle can be combated (e.g. include anti-scaling agents in the feed pretreatment), and the economic benefits of including the brine recycle are very significant.8
MSF: Continuous vs. Batch
The distillation step can be done in continuous or batch processes. Batch distillation is more versatile and often used when the product is in small amounts and very high purity. A continuous distillation is more efficient thermodynamically and economically for large amounts of material of constant composition, whereas batch distillation is more effective for small amounts of material of varying compositions. Since the plant is processing large amounts of seawater of relatively constant composition, the distillation system will utilize continuous distillation.9
MSF: Long Tube vs. Crossed Tube
Inside the evaporator stage, the condenser tube bundles may be arranged in either long tube or cross tube design. In the long tube arrangement, the tubes are aligned parallel to the direction of the flashing brine flow. The tubes go from stage to stage without water boxes. In the cross tube arrangement, the tubes are lined perpendicular to the flow of the flashing brine. Water boxes transfer brine between the stages.10 11 While the water boxes in crossed tube bundles allow for more customization, the long tube configuration allows for construction of large unit sizes, as there are manufacturing restrictions with cross tubes. Long tube design can easily accommodate a large number of stages, which lead us to choose long tube for our design.11 12
MSF: Venting System
Noncondensable gases exist in the process because of air leakages into stages under vacuum conditions and the release of dissolved gases from the heated brine. These gases are removed by venting the system. An air cooler section concentrates the gas before they are vented, and baffles direct the vapor and condensates out. There are two venting arrangements to consider: series and parallel. The system in series moves gas from one stage directly to the next, and the only loss in the system is vapor in the last stage (since some vapor is extracted with gases). This system is economical, however this configuration increases the amount of noncondensable gases passing from one stage to the next, and disrupting the venting system in one stage will affect the entire system. In parallel venting, gas is released through parallel takeoff pipes at each stage. Although this arrangement allows for venting at any stage to be controlled, the design and maintenance is more complex, and more vapor is lost than in the venting system in series. A third alternative is to use a combination of venting in parallel and in series, which is the common method in industry. For this process, our team chose to do venting in parallel and in series.12
Additional Energy Source Alternatives
Energy for the process can also be coupled with renewable energy sources to reduce the desalination plant’s carbon footprint. Energy integration can be direct or indirect. In direct integration, thermal waste heat from industrial sources provide thermal energy or pressure as energy sources. Direct integration is advantageous at the modular level, as it avoids energy losses with electricity conversion. Within indirect integration, there are modular integration and grid/utility-scale integration. Modular integration integrates the plant with wind turbines and other small-scale renewable power generators with mobile deployment. A “hybridization” of renewable power sources with natural gas power generation is also an option, and the coupling of the two provides a stable power source. Indirect renewable energy integration is growing as the cost of renewable energy continues to decreases and provides an economy of scale that direct integration does not, but there are several obstacles towards indirect integration, such as the plant’s finance and engineering the integration of the two systems.13 This distillation plant will be directly integrated with neighboring plants such as Chevron that produce thermal waste heat.
Product Treatment
Since the product is distilled water, it needs additional ions to a concentration of around 0.01% before going to the end user. The three alternatives considered were building a water treatment facility on site, simply adding the correct salts to the product stream and then sending the stream for bacterial treatment at a water plant, or simply sending the product distilled water for complete treatment to another facility. Due to the number of nearby water treatment plants and their demand for water, we elected to simply send the product to a nearby water treatment facility.
Design Constraints and Tradeoffs
While designing our process, we wanted to make sure that we met several outside constraints, including environmental, political, ethical, social, sustainability, health and safety constraints. First and most importantly, we wanted to ensure that the brine discharged back to the bay would not harm local marine life. Our process was designed around the constraint of diluting the exiting brine to within 0.2% salinity of background seawater. This is not only compliant with California law, but was also verified to be safe for surrounding marine life through our own outside research. We also addressed many of these considerations by deciding to pursue direct energy integration with the nearby Chevron plant in order to reduce environmental impact of our process. Additionally, we ensured that our plant would be located in an area that is densely populated and in great need of fresh drinking water. Finally, we addressed health and safety considerations by making sure we would be able to send our product distilled water to local treatment plants for proper potabilization.
Design Tradeoffs: Thermodynamics
Adjusting elements within this process is iterative, and optimization thereof required changing multiple parameters at once. An example of this is in tuning the temperature increase by heater E-100; if the temperature of the stream entering the vessels is too high, the heat exchange between the vapor product and the feed stream will not be realizable because it causes the feed to be too hot to cool and condense the product. Higher temperatures, however, provide greater thermodynamic driving forces for flashing. Setting the entering stream temperature at 120°C provides enough heat for substantial flash at 130 kPa. This is the only heater required until the very last flash stage, which is added to provide more heat for vaporization. This heater (E-105) increases the stream temperature by 15°C, and the vapor product from the last vessel increases the feed stream temperature by almost 30°C. The added energy increases vaporization by 3%, which is a substantial 2.2 million kg/h. The temperature of the streams entering and leaving each stage decreases with each additional flash stage such that the last vessel has the lowest temperature, according to realistic operating guidelines.
Design Tradeoffs: Energy Consumption, Environmental Impacts, and Product Optimization
Increases in the pressure drop across any of the valves in this final design causes a temperature cross in the heat exchangers unless a proper increase in pump energy is provided. There is thus a design tradeoff between lowering energy usage and increasing product yield. For example, lowering the operating pressure of the process dictates that the pressure difference used to flash seawater must be smaller, possibly decreasing the amount that is vaporized. The final design aims for a yield of 50 million gallons per day. The 18 stage model was chosen for its low annual utility cost. This is a reflection of environmental mindfulness because energy consumption is an ongoing aspect of the process and should be lowered as much as possible.
Mass and Energy Balances
The model of the desalination system assumes that: (1) the properties are uniform in each phase within a stage, (2) heat losses are negligible due to the small surface area to volume ratio for each stage and properly insulated units (heat losses to the surrounding vary from 2 to 5% of the total system energy), (3) the distillate is salt free since the boiling point of water is much lower than that of salt, (4) the subcooling or superheating effects on the system energy balances are negligible, (5) the only contaminant is sodium chloride (there are no non-condensable gases to consider, so no vapors are vented), and (6) the process has 3 evaporation chambers.
The major balances are that the feed is a mixture of the fresh seawater stream and the recycle stream, and that the brine stream exiting the last evaporation chamber is divided into the recycle stream and the waste stream. Within each stage, the change in mass of a phase over time is the balance between the net flow of that phase from the chamber and the rates of evaporation and condensation of water into and out of that phase. The equations governing the mass and energy balances on the system is displayed in Appendix C.
Optimization
As initiatives for reduced energy usage and increased environmental sustainability become more prevalent, efforts to minimize the utility cost in plants has increased to accommodate these incentives. Utilities are a continual source of expenditure and consumption, and a well-designed chemical process should seek to minimize this element. The annualized utility cost is a useful parameter to optimize given that its value depends on variations in energy costs; thus, annualized utility costs do not correlate with conventional indices of inflation, such as capital and labor costs. Additionally, utility costs, like other variable costs, can be minimized by improving the design or operational efficiency of the plant.
In optimizing our MSF process, the design variable of focus was the number of stages to use in our process. The performance measure was the annualized utility cost, which was the parameter that was minimized for optimization. The process is constrained by the product specifications, which are kept constant and serve as the standard for comparison between each model. These specifications are to produce 50 million gallons of desalinated liquid water per day.
One feasibility constraint in the process is the size of the heat exchangers, which must provide a large enough area for total condensation of the vapor stream coming out of the flash stages. In order to restrict the heat exchange area needed within each exchanger, the number of stages must be at least 11. Increasing the number of stages decreases the vapor flow rate at each, and decreases the heat exchange area required as well. An 11-stage process allows for appropriate heat exchange between the feed stream and the vapor product, according to the HYSYS economic analyzer. The optimization analysis thus was conducted incorporating only models with 11 or more stages.
The HYSYS economic analyzer was used to systematically optimize the process by finding the model with the lowest annualized utility cost. The performance parameters kept constant across each model were: the inlet composition, pressure, and temperature, the brine outlet temperature, the total product flow rate and composition, and the flash stage schematic. Table 1 displays the annualized utility cost for each model, and illustrates that a clear dip in the utility cost occurs in an 18 stage model. Thus, 18 is the optimal number of stages to use for our MSF distillation given utility costs as the optimized parameter.
Economic Evaluation
In order to evaluate our process from a fiscal standpoint, we used Aspen Process Economic Analyzer and Aspen cost estimator to determine capital and operating costs for our plant. These costs were calculated based on calculated equipment sizes, as well as energy requirements reported by our HYSYS model. The capital cost was found to be $485.2MM and annual total operating cost was found to be $144.7MM ($103.7MM of which was utilities). Annual plant revenue was estimated based on prices that water districts in the county currently pay for outside water sources, and was found to be $134.3MM.14
The NPV analysis we conducted assumed that the plant would take two years to build, would operate at half capacity during the first year of operation (year 3), and have a 20 year lifetime. We also assumed that our plant would be in operation 350 days per year. A constant discount rate of 12% was assumed to hold throughout the plant’s lifetime. The final NPV of this project is -$495.7MM. This is due to both the large capital cost of the plant and the annual loss of $10.4MM while in full operation. A graph of cumulative cash flow versus time can be found in Appendix E. Tax rates and depreciation do not directly impact the NPV of this project due to operating at a loss, however for future calculations it should be noted that we used a straight line depreciation schedule over a seven year period with a 40% tax rate.
Despite the current projections, it is important to consider the greater implications of running a desalination plant in the Bay Area. The population in the Bay Area is quickly growing, while California is experiencing harsher droughts each year. A consequence of this is the possibility of receiving government subsidies for our clean water product. The similarly scaled desalination plant in Carlsbad, CA operates at a loss as well, but receives significant government subsidies. Building this facility may be a strategic investment, especially since greater water scarcity is predicted in the future.
Sensitivity Analysis
We wanted to determine how sensitive NPV of our project is to the cost of capital, price of utilities and price we would be able to sell our clean water product at. We conducted this analysis by varying each of these variables independently between 0.5 times and 1.5 times the original value we calculated. As to be expected, NPV grows more negative with increasing capital costs and utility prices and decreasing water prices. Conversely, NPV becomes less negative as price of water rises and capital and utility costs decrease. A plot summarizing the effects of varying these costs can be found in Appendix E. NPV is most sensitive to the price at which we will be selling our clean water product. It is worth noting that NPV is more sensitive to utilities cost than it is to capital cost, which is why we focused on optimizing the cost of utilities. In future steps, trying to reduce utilities cost even more would be worth looking into to decrease the amount of money being lost on this project.
Conclusions and Final Recommendation
The final design achieves the project goal of producing 50 million gallons per day of distilled water at a yield of 13%, comparable to other similarly sized plants. An optimization of the number of MSF stages is realistic, and can be improved upon by taking into account other process parameters.
The proposed design was optimized around utility costs, comparing plants with 11 to 25 stages. The process is constrained by product specifications and by the thermodynamics of the heat exchange between the feed and the product streams. Energy conservation was achieved in using the seawater feed to condense the final product, as well as in all of the heat exchangers increasing the thermal energy of the seawater feed stream before it enters the flash stages.
In order to further optimize this process, other design parameters should be considered such as the brine recycle ratio, the cooler duty of the recycle stream, the pressure drop across each valve at the flash stages, and other economic factors. An optimization around maximizing NPV, for example, might be more comprehensive than that conducted regarding only utility costs. Tuning brine recycle rates could greatly improve process efficiency, and standardizing flash stage pressure drops would make operations more feasible and controlled.
Given our in depth economic analysis, this project would lose an average of $10.4MM per year, and has an NPV of -$495.7MM for a 20 year plant lifetime. From an economic perspective alone it would not be a good project to build. However, other factors need to be considered when building a project with enormous public value. In recent years California droughts have become more severe. Implementing a drought-proof supply of water now could avert a disaster later on. Even without a large disaster, having the desalination plant could also be expected to pay off in the future with the increasing price of water. Considerations such as these were the deciding factors in building the Carlsbad desalination plant. Since there are currently no desalination plants in the Bay area we recommend that preparations should be made to move forward with a desalination plant.
References
[1] Lyons J. Environmental Leader. Environmental Leader 2015. Available at: http://www.environmentalleader.com/2015/10/27/desalination-plants-to-double-by-2020. Accessed 2016.
[2] Fitzsimmons E. In California, Cities Braced to Cut Water by 10 to 35%. The New York Times 2015. Available at: http://www.nytimes.com/2015/04/09/us/in-california-cities-braced-to-cut-water-by-10-to-35.html. Accessed 2016.
[3] Salinity Times Series - USGS Water Quality of SF Bay. Salinity Times Series - USGS Water Quality of SF Bay. Available at: http://sfbay.wr.usgs.gov/access/wqdata/overview/examp/charts/salin.html. Accessed 2016.
[4] Reverse Osmosis vs. Multi Stage Flash Distillation: A Comparison Between Different Desalination Methods. Brighthub Engineering. Available at: http://www.brighthubengineering.com/power-plants/29621-comparison-between-the-reverse-osmosis-and-multi-stage-flash-distillation-methods/. Accessed 2016.
[5] Committee on Advancing Desalination Technology, Water Science and Technology Board, Division on Earth and Life Studies, National Research Council. Desalination: A National Perspective. Washington, D.C.:The National Academies Press, 2008.
[6] H. El-Dessouky. Fundamentals of salt water desalination, 1st Ed., Elsevier Science, 2002.
[7] Committee on Advancing Desalination Technology, Water Science and Technology Board, Division on Earth and Life Studies, National Research Council. Desalination: A National Perspective. Washington, D.C.:The National Academies Press, 2008.
[8] Guasp RE, Bello CP, Jara JG, Vega JP, Damien T, Winkel C, Smith HH. Desalination by distillation. Organization of American States website. oas.org. Accessed January 28, 2015.
[9] Batch Distillation. AE 335 Separation Processes website. http://prodpran.che.engr.tu.ac.th/AE335/AE335.html. Accessed January 26, 2016.
[10] Morris R. The development of the multi-stage flash distillation process: A designer's viewpoint. Desalination. 1993;93:57-68.
[11] Darwish MA. Developments in the multi-stage flash desalting system. Desalination. 1995;100:35-64.
[12] Wade N. Distillation plant development and cost update. Desalination. 2001;136:3-12.
[13] Desalination Technologies and the Use of Alternative Energies for Desalination. World Intellectual Property Organization website. http://www.wipo.int/export/sites/www/patentscope/ en/programs/patent_landscapes/documents/patent_landscapes/948-2E-WEB.pdf. Updated November 2011. Accessed January 23, 2016.
[14] California Turns to the Pacific Ocean for Water | MIT Technology Review.MIT Technology Review 2014. Available at: http://www.technologyreview.com/featuredstory/533446/desalination-out-of-desperation/. Accessed 2016.
Appendix A. Process Flow Diagram
Figure A1 Process Flow Diagram of the MSF System
Appendix B. HYSYS Simulation
The HYSYS model of the process is shown below.
Figure B1. The HYSYS model of the MSF distillation process.
Appendix C. Mass and Energy Balances
Below are equations that describe the mass and energy balances of the system.
In MSF system with brine recycle, the mass flow of the intake seawater I is mixed with the mass flow of the recycle brine R to create the feed seawater stream F. Since this model assumes no non-condensable gases, the feed stream is overall balanced by the brine waste stream W, the recycle brine stream, and the distillate stream D. The brine stream exiting the last evaporation chamber B is divided into the recycle and waste streams. The amount of salt that enters the series of evaporation chambers is the amount of salt that leaves (X is the salt mass fraction).
Within each stage, the change in mass of a phase is the mass flows of that phase into and out of the chamber and the rates of evaporation or condensation of the water into and out of that phase.
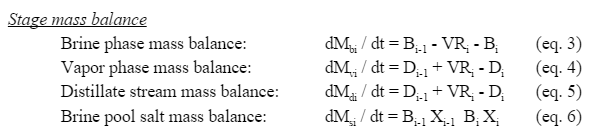
The energy balances are summarized below.