Design S1: Difference between revisions
| Line 77: | Line 77: | ||
===Appendix 5=== | ===Appendix 5=== | ||
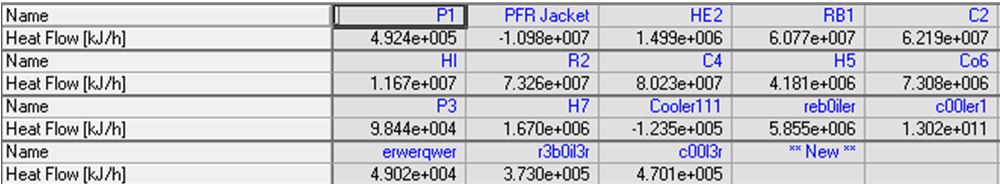
Energy Stream Summary | Energy Stream Summary | ||
[[File:Energy Stream Table.JPG]] | |||
===Appendix 6=== | ===Appendix 6=== | ||
HYSYS Simulation | HYSYS Simulation | ||
Revision as of 19:30, 14 March 2014
Executive Summary
The Chemical Intermediary Division ($1) of Evanston Chemical Corporation is interested in evaluating the economic benefit of adding 1,4 Butanediol (BDO) to its already robust offering of chemical intermediaries. It plans on producing 50,000 tonnes of 99.5%wt BDO using a modified Davy Process through a Gas Liquid Induction Reactor (GLIR) with a rare earth metal catalyst. The facility will be located in Lake Providence, LA , due to proximity to raw material productions sites and ease of transportation of the final product.
BDO is produced from Succinic Acid (SUC) by a hydrogenation reaction at very high pressures. This particular process uses a rare earth catalyst to because of the high conversion of SUC and the high selectivity toward BDO. Side products of the reaction include the highly valuable chemicals tetrahydrofuran (THF) and γ-butyrolactone (GBL), as well as water, n-butanol, and various other low value chemicals. The reaction occurs at 165oC and 150 bar, so special attention must be paid to reactor design to account for these extreme conditions.
Aspen HYSYS was used to model this process, and equipment sizes were calculated as specified by Towler. Equipment costs were estimated using Aspen Process Economic Analyzer (formerly known as Icarus). In order to meet the process specifications within the given time-frame, some simplifying assumptions were made that impose limits on the accuracy of the model; specifically, the GLIR was modelled using a conversion reaction. This means that the conversion remained unchanged at unideal operating conditions, which, clearly, is inaccurate. Additionally, only one reaction was modelled; in real world conditions, dozens of reactions would occur within the reactor, which would have a potentially massive effect on the accuracy of this model. Despite these limitations, the possibility of producing BDO for sale in the open market should be investigated because of the extremely encouraging economic potential of this endeavor.
Economic analysis of this process indicates a 10 year NPV of $163M, a 20 year NPV of $258M, and a total capital cost of $17.5M. The variable operational costs are approximately $131M. At a selling point of $3/kg for BDO and $35/kg for GBL, the total revenue will exceed $190M/yr, so it is strongly recommended the ECC pursue this opportunity.
Introduction
Project Justification
1,4 Butanediol is an important organic chemical used mainly as an intermediate for the production of Tetrahydrofuran (THF), Gamma-Butyrolactone (GBL) and Polybutylene Terephthalate (PBT). In 2011, the global market for BDO reached 1,725.0 kilo tons. Current projections suggest that worldwide BDO consumption will grow to 2550 kilo tons by 2017. The Technology Division of Evanston Chemicals has requested a preliminary design and economic evaluation on the feasibility of producing 1,4 Butanediol from a biomass derived succinic acid.
Technology Review
BDO can be produced by hydrogenation of succinic acid in the presence of a catalyst, at high temperatures and pressures (approximately 150-200°C and 20,000 kPa). However, to the best of the authors’ knowledge, there does not exist a means to produce only BDO. Instead, a mixture of THF, GBL, and BDO is produced. The choice of catalyst is the major factor in controlling yields and selectivity of a certain product. Current research suggests that bimetallic catalysts have a stronger activity and lead to higher conversion to BDO. TiO supported 2%Pd/X%Re catalyst and Carbon supported 2%Ru/4%Re catalyst both have been shown to be particularly effective. It is interesting to note that both catalysts contain Re. Research suggests that the synergy between Re and Pd/Ru leads to higher selectivity for BDO. Additionally, the chosen catalyst for this study was a 0.4% Fe, 1.9% Na, 2.66% Ag, 2.66% Pd, 10.0% Re on 1.5 mm carbon support. Taken from an ISP Investments patent from 2011, this catalyst has exceptionally high yields, upwards of 99.7% conversion of succinic acid, as well as high yields of BDO. In total, the percentage conversions of succinic acid to various products are: BDO: 85.51 wt % GBL: 2.04 wt % THF: 9.28 wt % Butanol: 2.87 wt % The lifetime of the catalyst is approximately 5 years. Furthermore, multiple reactor types have been discussed in the literature, including CSTRs and PFRs. However, the literature also shows that gas liquid induction reactors are the best for hydrogenation reactions in industry. They are preferred for their safety with respect to explosive gas phase reactants and complete utilization of the gas phase reactant; being especially useful for expensive inputs. These reactors use energy efficient impellers for gas induction and dispersion along with special recovery equipment to prevent waste of the gas phase, thus GLIR was chosen for this study.
Design Basis
The plant has been proposed to be built in Lake Providence, Louisiana. This is due primarily to its proximity to suppliers of succinic acid in the south east region, as well as close to distributors by way of the Mississippi River and the Gulf of Mexico. Additionally, the plant has been designed to have a capacity of approximately 50MM kg/yr of BDO production. This will require feeds of compressed hydrogen gas, and an aqueous feed of water and succinic acid, in a 50/50 wt % composition.
Technical Approach
The primary tool used to model the plant process was Aspen HYSYS. Because of the non-idealities involved in the NRTL HYSYS fluid package, an additional fluid package was created in Aspen. Additionally, the GLIR reactor chosen was modeled as a conversion reactor. Selectivity and yield of the reactor was modeled based off the specific data from the ISP Investments patent (discussed in Introduction: Technology Review). Although this provided very accurate modeling, the operating pressure and temperature were required to be held constant. This prevented any optimization within the reactor with respect to these operating conditions. The distillation columns were modeled as fractional distillation columns, with bottoms or distillate flow rate, and component recovery as active constraints. Additionally, Aspen Energy Analyzer was used to create the heat exchanger network, which can be seen in the Appendix. The economic analysis was performed in Aspen ICARUS.
Figure 1. Screenshot of Aspen HYSYS process simulation. As mentioned above, this plant will produce technical grade BDO and GBL, but the THF stream will be treated as waste. Originally, attempts were made to purify the THF stream in order to sell it; this process produces approximately 5M kg/yr, and at a selling point of ~$3.00/kg, this would generate an additional $15M/yr in revenue. However, the stream containing THF also contains water and n-butanol. As shown in Appendix XX, THF and water have a pressure dependent azeotrope; Figures XX1 and XX2 show that the azeotrope can be broken by pressurizing the stream after initial separation and atmospheric pressure. However, the presence of n-butanol makes separation of THF and water impossible; butanol and water have a tight azeotrope that cannot be broken simply by pressurizing the stream. Instead, absorption or ion exchange must be used to purify the stream. After extensive efforts were made to purify the THF, it was decided that the undertaking would not be profitable. In future iterations of this project, redoubled efforts would be made to force separation of THF to generate large revenues.
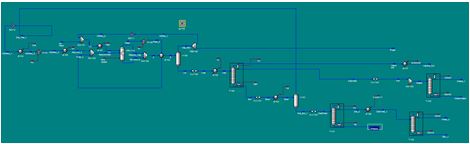
Process Flow Diagram & Flow Sheets
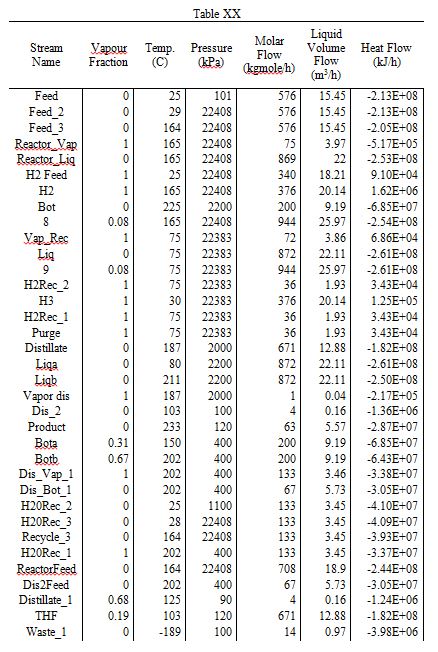
Below is listed a complete flow sheet, with data taken from Aspen HYSYS.
Table 1
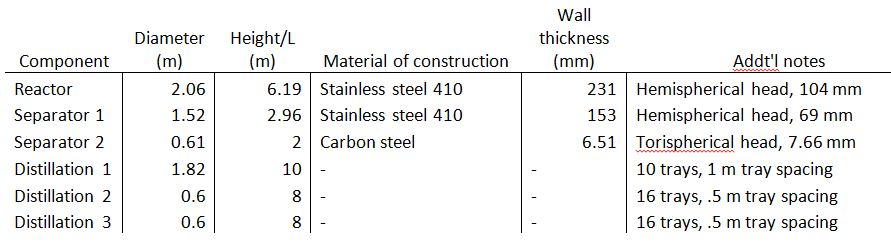
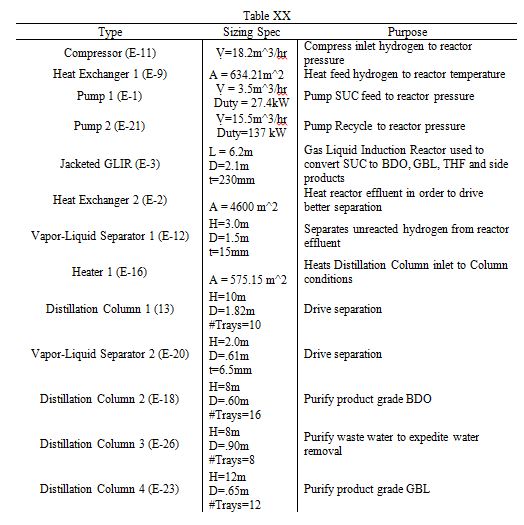
A detailed sizing list of the relevant components is listed below. Of particular interest are the distillation columns, which have heights between 8 and 12 meters. Furthermore, the reactor has a length of 6.2 meters and a diameter of 2.1 meters. A more complete table of sizing and sizing methodologies is listed in the Appendix.
Table 2
Economic Analysis
Table 3
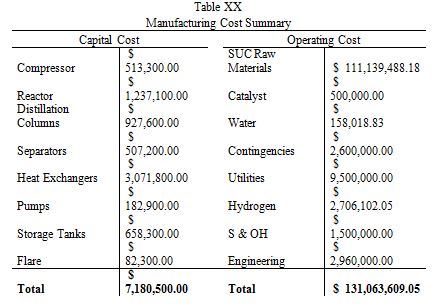
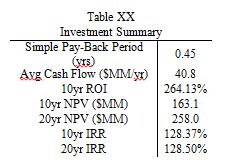
As shown clearly in Table 3, this plant has extremely encouraging economic potential, with an average yearly cash flow of $40.8M, a simple payback period of less than 6 months, and a breakeven point of approximately 9 months. These capital projections assume a 2 year construction time, 38% tax rate, 50% capacity in the first year of production, and a 7 year MACRS depreciation method. Revenue is split into two streams; main product (BDO) and by-product (GBL). The main product revenue is approximately $150M/yr, while the by-product revenue is $40M/yr, despite producing nearly 50 times less GBL than BDO. In future iterations of this design, it may be worthwhile to examine if it is more profitable to convert all of the succinic acid directly to GBL instead of producing the intermediary, BDO. If the economic returns seem astronomical, it is because they are; no economic investment actually produces these kinds of returns. The extremely favorable prediction could stem from several sources of error; supply and demand, bulk pricing, waste removal, and side reactions. The yearly supply of BDO is approximately 1M metric tons, meaning that this plant would produce 5% of the world’s supply. If the supply of BDO outpaced the demand, the selling price would fall, cutting into main product revenues. Additionally, the estimate of GBL selling price was based off a per kg price; if bulk pricing were used, the selling price of GBL would be between 40-50% lower than the price used. In addition to economic discontinuities, the problem of waste removal was never fully solved. Based on past estimates, waste removal could cost as must as $10M/year, further reducing the profitability of this plant. Lastly, the process of BDO synthesis was simplified for modelling purposes. In actual production, many side reactions would occur; driving down conversion and increasing separation costs.
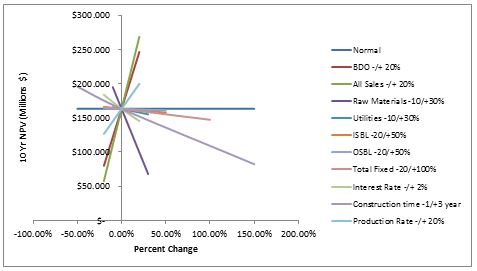
A sensitivity analysis was performed on all important variables.
Conclusion
To conclude, this project is highly feasible, and our company stands to make a very high profit from the construction of this plant. Based on a two year construction time, a 38% tax rate, and 50% production over the first year, our simple payback period would be 0.45 years, with yearly revenue of over $190M. We strongly recommend Evanston Chemical to move forward with this facility. However, there are key next steps to be considered. First, a more detailed reaction scheme for the reactor is required. This scheme must be able to handle variable operating pressure and temperature, as well as contain a more detailed and complete incorporation of side reactions. Namely, the reaction of GBL to BDO is a necessary consideration. Moreover, a fully life cycle analysis and environmental health and safety analysis will be required. The removal of waste will also need to be addressed. Additionally, profits may be increased by performing a plant wide optimization project. Also, the assumption of a 5 year catalyst lifetime will need to be addressed in more detail, as the high cost of this component will greatly affect the bottom line. Another factor which may affect the overall profitability is the flocculating prices of rare metals, which make up the catalyst. Also, the market price of these commodity chemicals will almost certainly be affected by a 10% influx of global supply. Finally, while we have planned on also selling GBL, looking into purifying the THF will also be profitable.
Appendices
Appendix 1
Sizing and construction information.
Appendix 2
Appendix 3
Appendix 4
Appendix 5
Appendix 6
HYSYS Simulation
Appendix 7
Stream Summary Table
Appendix 8
Summary of HYSYS stream compositions.
Appendix 9
Economic analysis summary
Appendix 10
Assorted economic data
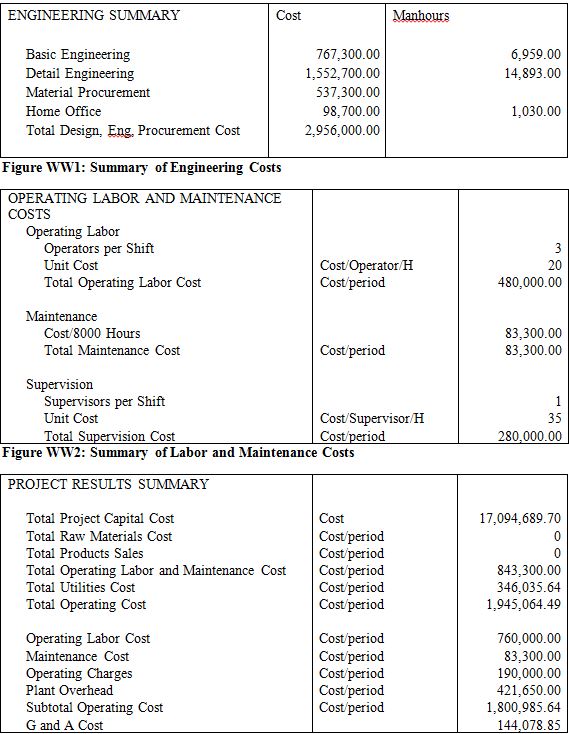
ENGINEERING SUMMARY Cost Manhours
Basic Engineering 767,300.00 6,959.00
Detail Engineering 1,552,700.00 14,893.00
Material Procurement 537,300.00
Home Office 98,700.00 1,030.00
Total Design, Eng, Procurement Cost 2,956,000.00
Figure A1: Summary of Engineering Costs OPERATING LABOR AND MAINTENANCE COSTS
Operating Labor
Operators per Shift 3
Unit Cost Cost/Operator/H 20
Total Operating Labor Cost Cost/period 480,000.00
Maintenance
Cost/8000 Hours 83,300.00
Total Maintenance Cost Cost/period 83,300.00
Supervision
Supervisors per Shift 1
Unit Cost Cost/Supervisor/H 35
Total Supervision Cost Cost/period 280,000.00
Figure A2: Summary of Labor and Maintenance Costs PROJECT RESULTS SUMMARY
Total Project Capital Cost Cost 17,094,689.70
Total Raw Materials Cost Cost/period 0
Total Products Sales Cost/period 0
Total Operating Labor and Maintenance Cost Cost/period 843,300.00
Total Utilities Cost Cost/period 346,035.64
Total Operating Cost Cost/period 1,945,064.49
Operating Labor Cost Cost/period 760,000.00
Maintenance Cost Cost/period 83,300.00
Operating Charges Cost/period 190,000.00
Plant Overhead Cost/period 421,650.00
Subtotal Operating Cost Cost/period 1,800,985.64
G and A Cost 144,078.85
Figure A3: Overall Summary of Project Results Appendix 10. VLE Data
Figure A4: Water vs THF VLE at 100 kPa Figure A5: Water vs THF VLE at 2000 kPa
Figure A6: Water vs Butanol VLE at 100 kPa Figure A7: Water vs Butanol VLE at 2000 kPa
Appendix 11
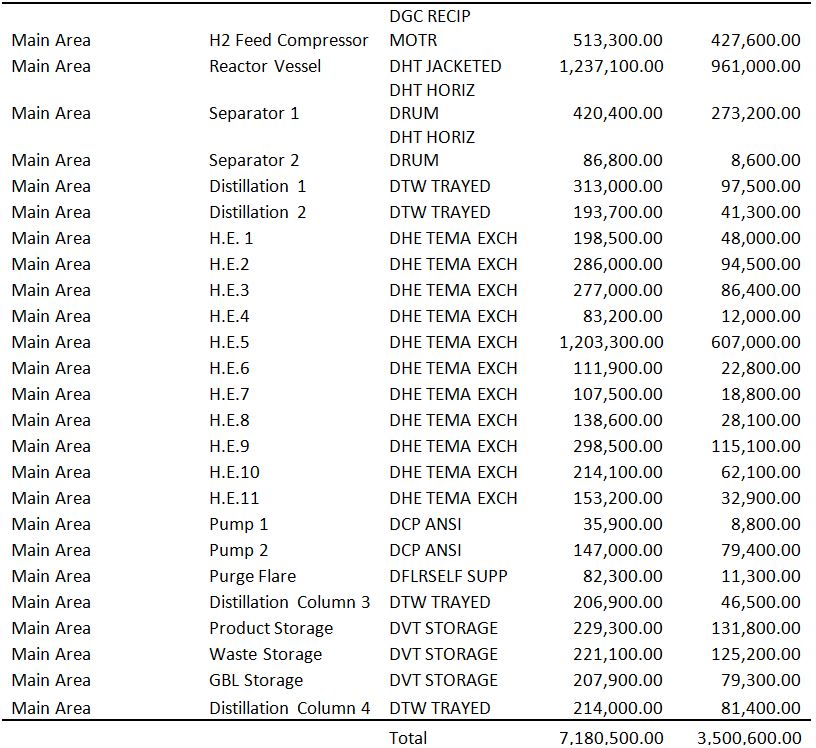
Project capital summary. PROJECT CAPITAL SUMMARY Total Cost Design, Eng, Procurement Construction Material Construction Manhours Construction Manpower Construction Indirects
Purchased Equipment Cost 3,562,500.20 3,562,500.20
Equipment Setting Cost 48,033.10 1,591.00 48,033.10
Piping Cost 1,839,521.60 1,300,326.60 18,056.00 539,194.80
Civil Cost 352,120.60 183,510.60 7,104.00 168,610.10
Steel Cost 71,926.20 58,874.10 493 13,052.10
Instrumentation Cost 1,109,755.50 924,111.60 6,171.00 185,644.00
Electrical Cost 684,348.20 596,287.40 3,084.00 88,060.80
Insulation Cost 594,591.20 362,250.20 10,562.00 232,341.10
Paint Cost 123,535.10 34,906.60 4,037.00 88,628.50
Other Cost 5,357,000.50 2,956,000.20 719,800.10 1,681,200.10
Subcontracts Cost 0
G and A Overheads Cost 323,619.90 0 232,277.00 40,906.90 50,436.00
Contract Fee Cost 597,333.90 174,404.00 159,496.90 117,975.60 145,457.40
Escalation Cost 0 0 0 0 0
Contingencies Cost 2,639,571.20 563,472.70 1,464,181.20 274,040.50 337,876.80
0
Total Project Cost Cost 17,303,857.20
Adjusted Total Project Cost Cost 17,094,689.70
Appendix 12
Heat exchanger network.
Appendix 13
Reaction mechanism summary