Materials of construction: Difference between revisions
KatieJohnson (talk | contribs) hardness vs tensile strength picture |
KatieJohnson (talk | contribs) case study |
||
| Line 74: | Line 74: | ||
==Inorganic Nonmetals== | ==Inorganic Nonmetals== | ||
Inorganic nonmetals include glass, stoneware, brick and cements.<ref name="peters" /> Ceramics are generally stronger than other materials, especially at higher temperatures, they are much more brittle.<ref name="ulrich" /> Glass is good for corrosion resistance; stoneware is generally corrosive-resistant with more strength, but has poor thermal conductivity.<ref name="peters" /> | Inorganic nonmetals include glass, stoneware, brick and cements.<ref name="peters" /> Ceramics are generally stronger than other materials, especially at higher temperatures, they are much more brittle.<ref name="ulrich" /> Glass is good for corrosion resistance; stoneware is generally corrosive-resistant with more strength, but has poor thermal conductivity.<ref name="peters" /> | ||
=Case Study= | |||
Chlorobenzene is produced by reacting liquid benzene with gaseous chlorine. The reaction takes place at 328K and 2.4 bar. If both the feeds are at 293K and atmospheric pressure, what are appropriate materials for the inlet piping and the reactor? (Adapted from ''Chemical Engineering Design'' <ref name="towler" />). | |||
Both benzene and dry chlorine are not corrosive and therefore, carbon steel can be used as the inlet piping. Note that if the gaseous chlorine is actually wet chlorine, it becomes very corrosive to most metals and a plastic should likely be used. While the reactor is at higher pressure than atmospheric pressure, it is well below the maximum allowable stress for all common materials <ref name="towler" />. However, a side product of the reactor is HCl which is corrosive. Likely, the HCl concentration will not be high enough to corrode the material but this should be investigated further. If concentration is >50% either in the reactor or later in the process, another material such as a plastic should be | |||
=References= | =References= | ||
<references/> | <references/> | ||
Revision as of 21:37, 1 March 2015
Author: Katie Johnson [2015]
Stewards: Jian Gong and Fengqi You
Material Properties
There are several properties of a material that can affect its suitability for the design. Before choosing a material, the designer should be aware of the following properties. Note that these properties for different common materials are often already collected and are available in various forms from manufacturers or in various textbooks.
Tensile Strength
The tensile strength, or tensile stress, of a material is the maximum amount of stress it can withstand before fracture. Proof stress, or yield stress, is similar, but measures that maximum amount of stress a material can withstand before deformation becomes permanent. Figure 1 below demonstrates where tensile strength (point u) and yield stress (point y) lay on the stress-strain curve for a material. There are standard tensile tests that measure tensile strength; however, strength is a common material property that is often already tabulated. [1]

In addition to considerations such as the pressure of the process, there are often guidelines that specify maximum allowable stress. One such set of guidelines is laid out by ASME in their Boiler and Pressure Vessel Code.[1] This should be consulted while designing pressure vessels. There are also equations that can estimate these values. The maximum pressure that a cylindrical vessel can withstand is given by the following equations, where t is shell thickness, p is pressure, R is the inside vessel radius, and S is the allowable tensile stress:
There are tabulations of S for various metals found in Perry’s Handbook. [3]
Modulus of Elasticity
The modulus of elasticity of a material, sometimes called its stiffness, measures the amount a material deforms when a certain amount of stress is placed on it. This measure applies when elastic deformation occurs, that is, when all deformation is reversible and is linearly proportional to stress.[4] In Figure 1, the modulus of elasticity for the material would apply between the origin and point y. This is important because it measures the resistance of a material to bending and buckling.[1]
Ductility
Ductility measures the amount a material will deform before it fractures.[1] The equation for ductility is as follows:
When a material has very low ductility it is defined as brittle. For example, in Figure 1 above, point f will be much closer to point u for a brittle material than for a ductile material. Brittle materials undergo very little deformation before they fracture, which means that in processes, there can be very little warming before a rupture. Some materials have a ductile-brittle transition points at low temperature. While these materials generally exhibit ductile properties, at low enough temperatures, they will not deform and will exhibit brittle fracture [5]
Hardness
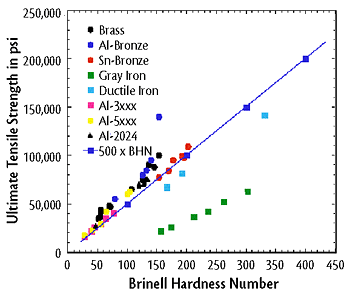
The material’s ability to resist plastic deformation such as dents.[1] There are many simple and relatively inexpensive tests, such as Rockwell Hardness Tests and Brinell Hardness Tests, which can determine the hardness of a material. It is useful to know the hardness of a material because it can be used to predict other mechanical properties such as tensile strength and can often be easier to determine. [4] Figure 2 below shows an example of the correlation between Brinell hardness number and tensile strength.
Fatigue Resistance
Fatigue is failure of a material that can occur when there is cyclic loading on equipment, for example, in pumps. It can also occur if there are cycles of temperature or pressure.[1] When there is cyclic loading, failure can occur at lower stress levels than the normal tensile strength. Fatigue failure is generally very similar to brittle failure with very little plastic deformation. [4]
Other considerations
There are many other properties to consider while selecting a material. For example, creep is the amount a material deforms while it is under constant tensile stress over long periods of time and can especially be a problem for metals at high temperatures. Other considerations include the ease of fabrication, including welding ability and flexibility, the availability and cost of material, thermal conductivity (which is especially important for equipment like heat exchangers), electrical resistance, and magnetic properties for certain cases. [1]
Process Considerations
Before choosing a material for a process, basic information must be collected, including temperature, pressure, and chemicals involved. The properties could affect the choice of materials.
Process Temperature and Pressure
In addition to knowledge of the average temperature a process with operate at, the engineer must be aware of the maximum and minimum temperature that could occur. While picking materials, the effect of temperature of material properties must be considered. Higher temperatures generally decrease the tensile strength and elastic modulus of metals.[1]
Additionally, very low temperatures can cause some materials to brittle fracture. Therefore, if the minimum process temperature is below the minimum allowable temperature for a material, a different material (such as low temperature carbon steel) must be selected. Note that the expected, maximum, and minimum environmental conditions should be considered in addition to internal conditions.
Similarly, the maximum and minimum pressures must be examined in relation to material properties. Different materials have different tensile stresses which affect that maximum pressure that can be used. When internal pressure is less than external pressure (that is, the process operates in a vacuum), either materials with higher allowable tensile stresses should be used or thickness should be increased.
Corrosion
If a process included certain corrosive chemicals such as oxygen, special allocations must be made for the materials. Additionally, environmental conditions such as salt from a nearby ocean should be considered. If corrosion is expected, the engineer should consider this in the material selection. This could involve picking a material that is naturally corrosive-resistant or by coating the inside of the pipe of equipment. These coatings can be made of paint or other organic coatings, especially for resistance to atmospheric corrosion.[1] For internal protection, materials can be lined with rubber, glass, stainless steel or various polymers.[3] [7]
Cycling
Cycling occurs when a certain aspect of a process (temperature, pressure, or material levels) continually cycles between high and low levels. Cycling puts additional stress on the system and should be considered when selecting materials.[4]
Common Materials
Metals
Carbon steel and stainless steels are some of the most common metals used in construction. Carbon steel is an alloy between carbon and iron. Also known as mild steel, carbon steel is one of the most commonly used engineering materials. It is favored because it is relatively cheap and widely available. It also has good tensile strength and ductility. However, carbon steel is not generally resistant to corrosion which can be an issue in many environments. When corrosion is expected, stainless steel is often favored. Stainless steel, especially with higher levels of chromium, is more resistant to corrosion.[1] Stainless steel is also a better choice for low temperatures, as it has a minimum rating of -425 F, as opposed to the minimum rating of carbon steel of -50 F. Stainless steels are also a better choice than carbon steel when temperatures above 1000 F are expected.[8]
Other options include nickel and alloys, including Monel, a nickel-copper alloy.[3] These are also corrosion-resistant to sulfuric and hydrocholor acids and salt water. Nickel-chromium alloys are good to chemical resistance at high temperatures.[7] Copper and alloys have the advantage of corrosion resistance and good thermal conductivity. Thus, copper is often favored for heat transfer equipment.[3] Aluminum and its alloys are more moderately priced than copper metals, are lightweight, and are better for low temperatures than carbon steel, however, they have lower strength and can also be susceptible to corrosion. [3] Aluminum is often appropriate for cryogenic operations. [7]
Plastics
Plastics are becoming more commonly used when corrosion is expected. Plastics are also favored because they are inexpensive. However, they have low strength compared to metals.[3] Plastics can be subdivided into several categories. The first of these is thermoplastic materials, which soften with increasing temperature. PVC falls within this category, and is the most commonly used thermoplastic material in chemical plants. The second category is thermosetting materials, which have a more rigid structure due to cross-linking. Rubber is also often used in linings for tanks and pipes.[1]
Inorganic Nonmetals
Inorganic nonmetals include glass, stoneware, brick and cements.[5] Ceramics are generally stronger than other materials, especially at higher temperatures, they are much more brittle.[3] Glass is good for corrosion resistance; stoneware is generally corrosive-resistant with more strength, but has poor thermal conductivity.[5]
Case Study
Chlorobenzene is produced by reacting liquid benzene with gaseous chlorine. The reaction takes place at 328K and 2.4 bar. If both the feeds are at 293K and atmospheric pressure, what are appropriate materials for the inlet piping and the reactor? (Adapted from Chemical Engineering Design [1]).
Both benzene and dry chlorine are not corrosive and therefore, carbon steel can be used as the inlet piping. Note that if the gaseous chlorine is actually wet chlorine, it becomes very corrosive to most metals and a plastic should likely be used. While the reactor is at higher pressure than atmospheric pressure, it is well below the maximum allowable stress for all common materials [1]. However, a side product of the reactor is HCl which is corrosive. Likely, the HCl concentration will not be high enough to corrode the material but this should be investigated further. If concentration is >50% either in the reactor or later in the process, another material such as a plastic should be
References
- ^ a b c d e f g h i j k l m Towler, G.P. and Sinnot, R. (2012). Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. Elsevier.
- ^ Engineering Archives Website. Stress Strain Diagram. http://www.engineeringarchives.com/les_mom_stressstraindiagram.html
- ^ a b c d e f g G.D. Ulrich, A Guide to Chemical Engineering Process Design and Economics, Wiley: New York, 1984.
- ^ a b c d e Callister, William and Rethwisch, David. Materials Science and Engineering, Wiley: New York, 2011.
- ^ a b c M.S. Peters, K.D. Timmerhaus, Plant Design and Economics for Chemical Engineers, 5th Ed., McGraw-Hill: New York, 2003.
- ^ Industrial Heating Website. Relationship between hardness and strength. http://www.industrialheating.com/articles/84495-engineering-concepts-relationship-between-hardness-and-strength?v=preview
- ^ a b c R.T. Turton, R.C. Bailie, W.B. Whiting, J.A. Shaeiwitz, Analysis, Synthesis, and Design of Chemical Processes, Prentice Hall: Upper Saddle River, 2003.
- ^ L.T. Biegler, I.E. Grossmann, A.W. Westerberg, Systematic Methods of Chemical Process Design, Prentice-Hall: Upper Saddle River, 1997.