Desalination - Team E: Difference between revisions
| Line 186: | Line 186: | ||
[[File:Appendix_2.png|center]] | [[File:Appendix_2.png|center]] | ||
==Appendix 3: Mass and Energy Balance Tables== | ==Appendix 3: Mass and Energy Balance Tables== | ||
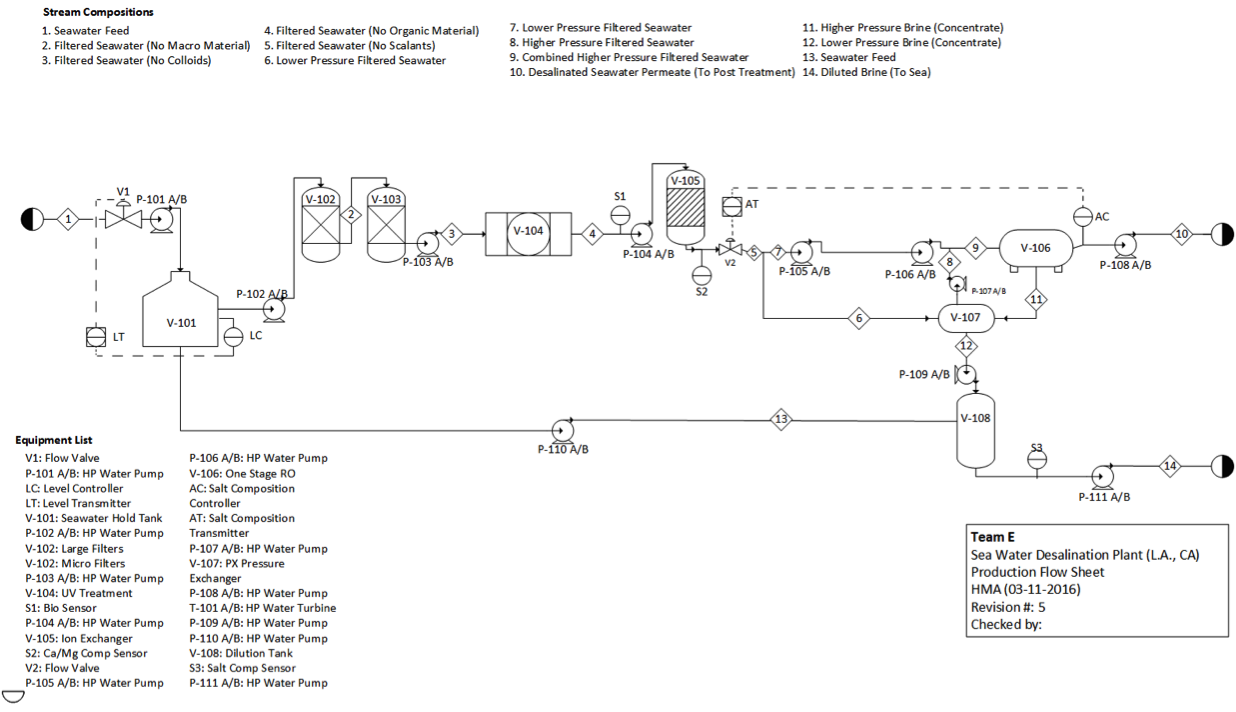
'''Table 1'''. Mass balance on 1 stage 5 element RO system, where "out" is the sum of all the concentrate and permeate. | |||
[[File:Appendix_3_T1.png|center]] | [[File:Appendix_3_T1.png|center]] | ||
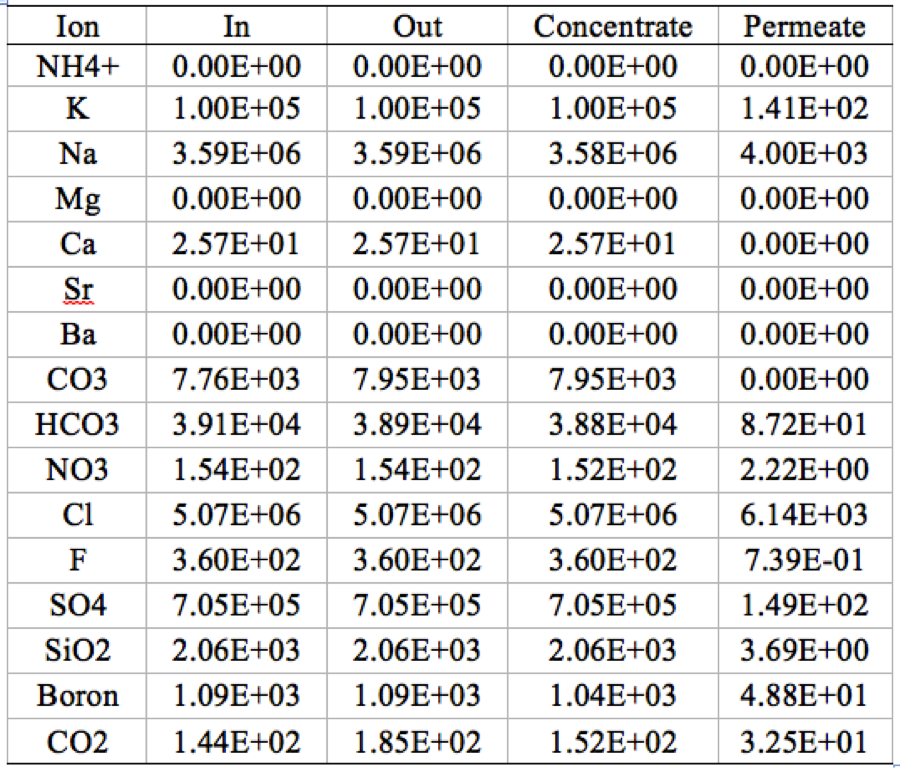
Table 2. Mass balance on 1 stage 1 element RO system, where "out" is the sum of all the concentrate and permeate. | '''Table 2'''. Mass balance on 1 stage 1 element RO system, where "out" is the sum of all the concentrate and permeate. | ||
[[File:Appendix_3_T2.png|center]] | [[File:Appendix_3_T2.png|center]] | ||
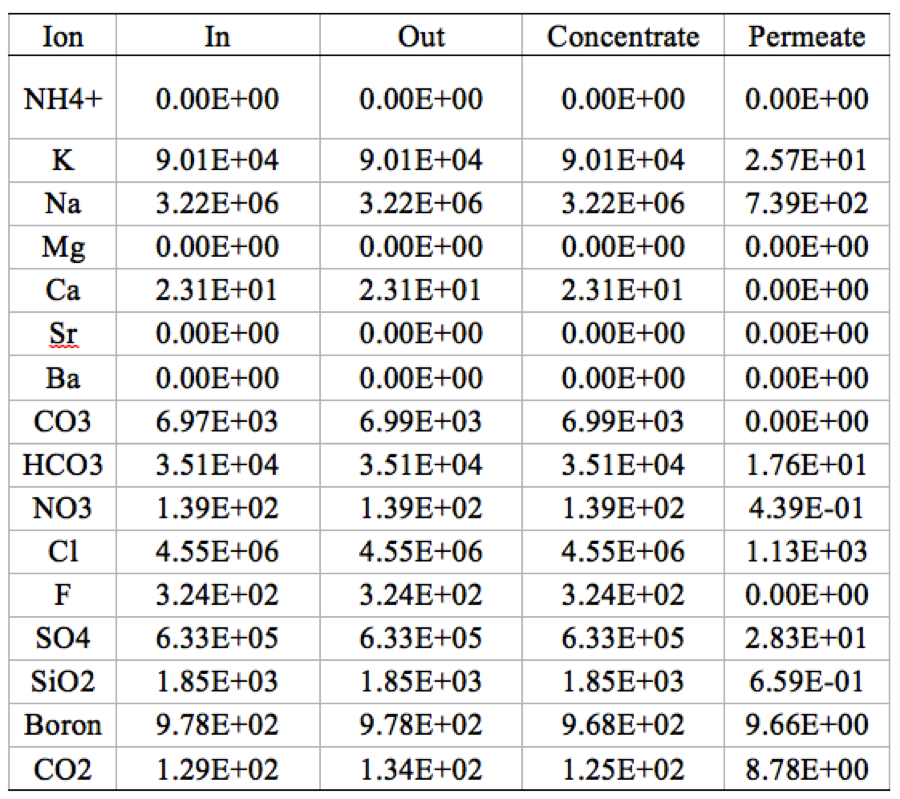
Table 3. Mass balance on 2 stage 5,5 element RO system, where "out" is the sum of all the concentrate and permeate. | '''Table 3'''. Mass balance on 2 stage 5,5 element RO system, where "out" is the sum of all the concentrate and permeate. | ||
[[File:Appendix_3_T3.png|center]] | [[File:Appendix_3_T3.png|center]] | ||
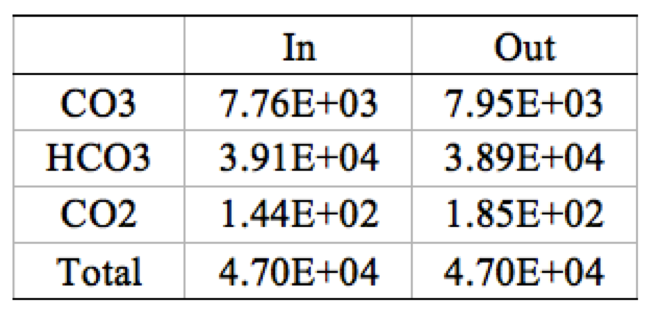
Table 4. 1 stage 5 elements CO<sub>3</sub>, HCO<sub>3</sub>, and CO<sub>2</sub> ion mass balance | '''Table 4'''. 1 stage 5 elements CO<sub>3</sub>, HCO<sub>3</sub>, and CO<sub>2</sub> ion mass balance | ||
[[File:Appendix_3_T4.png|center]] | [[File:Appendix_3_T4.png|center]] | ||
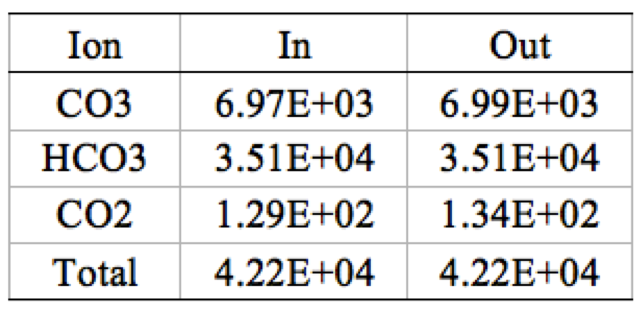
Table 5. 1 stage 1 element CO<sub>3</sub>, HCO<sub>3</sub>, and CO<sub>2</sub> ion mass balance | '''Table 5'''. 1 stage 1 element CO<sub>3</sub>, HCO<sub>3</sub>, and CO<sub>2</sub> ion mass balance | ||
[[File:Appendix_3_T5.png|center]] | [[File:Appendix_3_T5.png|center]] | ||
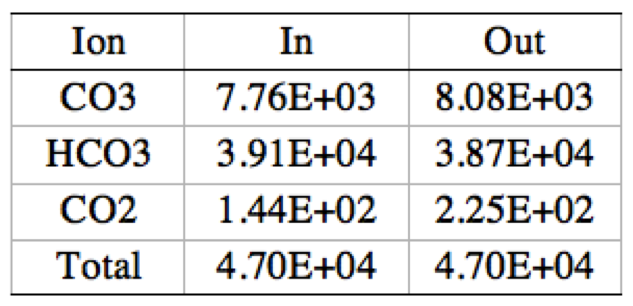
Table 6. 2 stages 5,5 elements CO<sub>3</sub>, HCO<sub>3</sub>, and CO<sub>2</sub> ion mass balance | '''Table 6'''. 2 stages 5,5 elements CO<sub>3</sub>, HCO<sub>3</sub>, and CO<sub>2</sub> ion mass balance | ||
[[File:Appendix_3_T6.png|center]] | [[File:Appendix_3_T6.png|center]] | ||
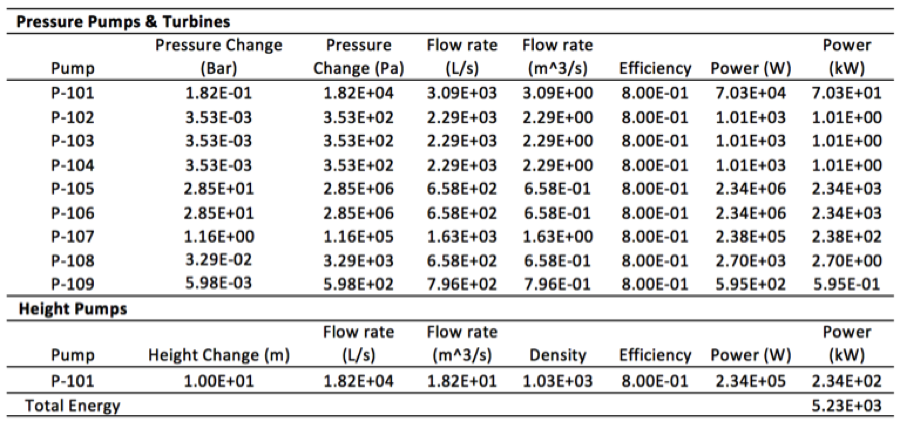
Table 7. Energy balance on 1 stage 5 elements RO system | '''Table 7'''. Energy balance on 1 stage 5 elements RO system | ||
[[File:Appendix_3_T7.png|center]] | [[File:Appendix_3_T7.png|center]] | ||
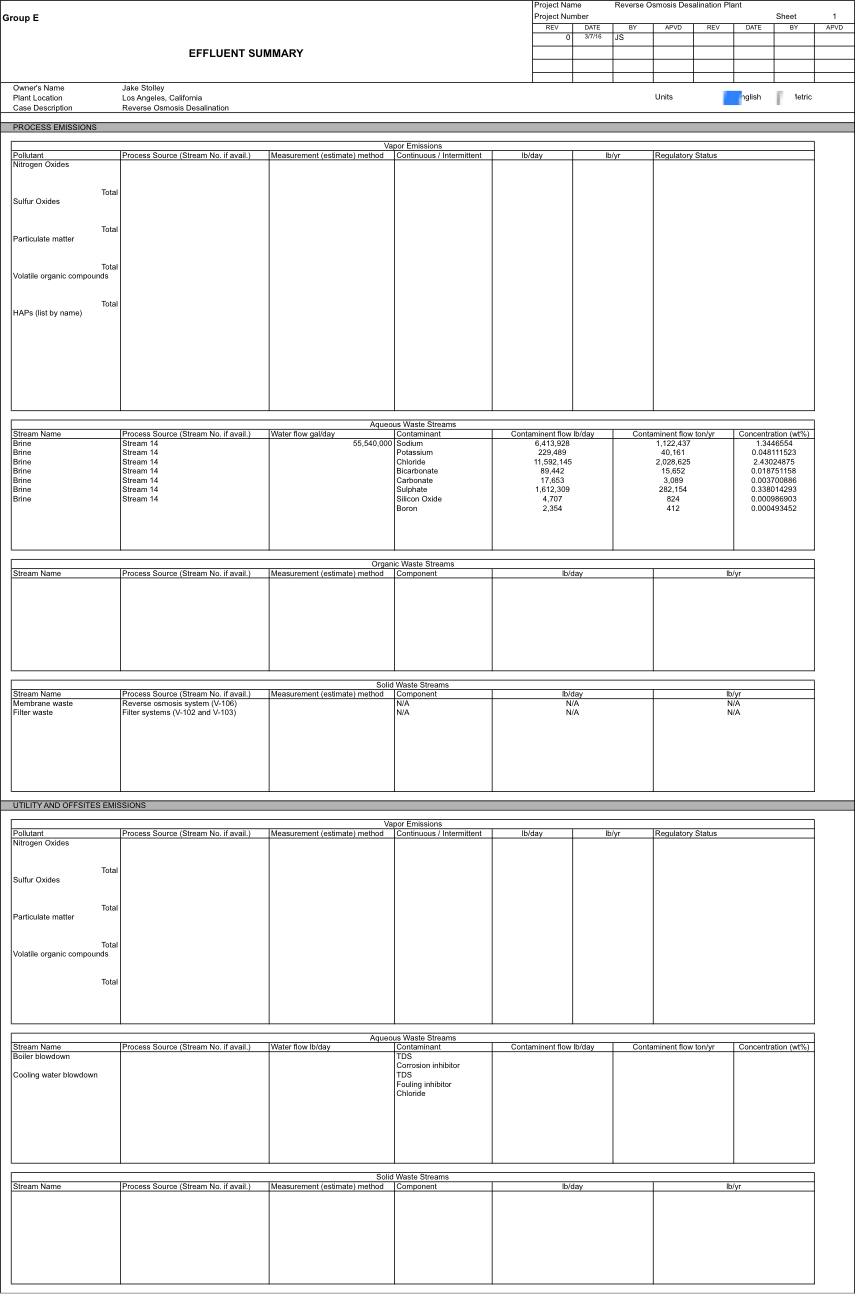
==Appendix 4: Effluent Summary== | ==Appendix 4: Effluent Summary== | ||
[[File:Appendix_4.png|center]] | [[File:Appendix_4.png|center]] | ||
| Line 240: | Line 240: | ||
==Appendix 7: ROSA Simulation Outputs== | ==Appendix 7: ROSA Simulation Outputs== | ||
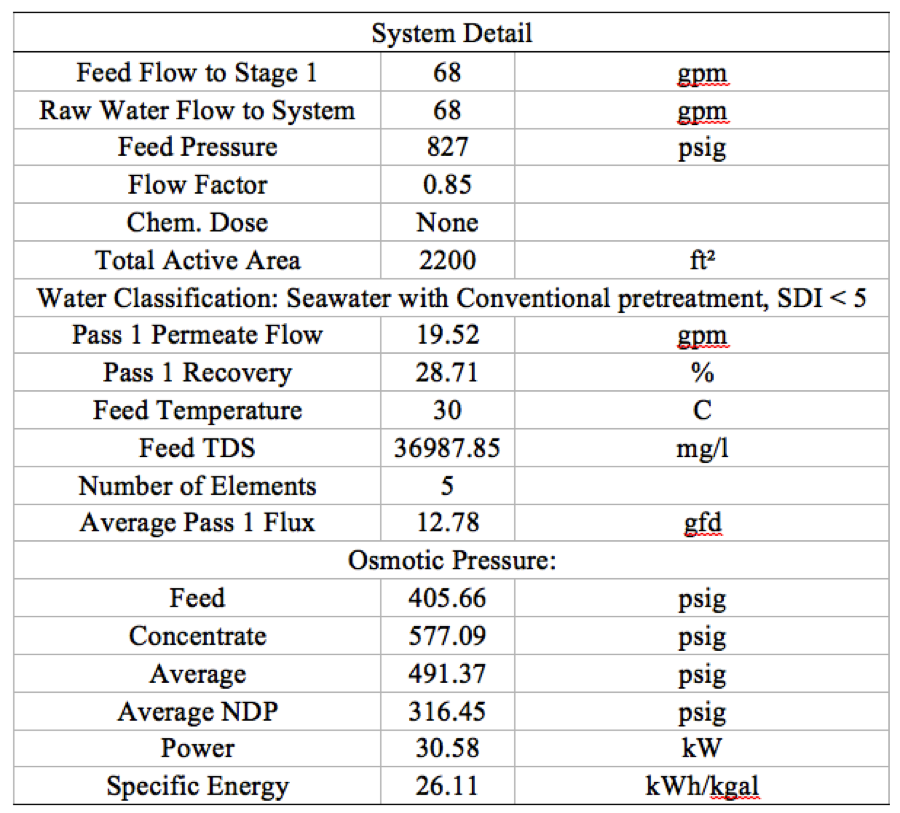
Table 1. System detail of 1 stage 5 elements RO system | '''Table 1'''. System detail of 1 stage 5 elements RO system | ||
[[File:Appendix_7_T1.png|center]] | [[File:Appendix_7_T1.png|center]] | ||
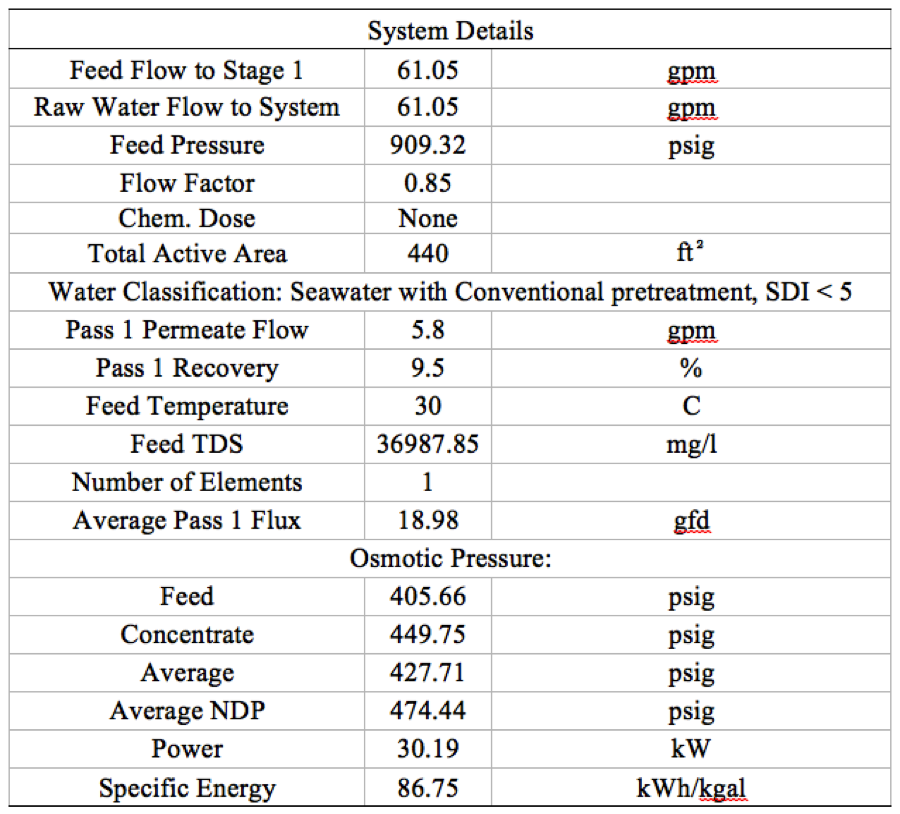
Table 2. System detail of 1 stage 1 elements RO system | '''Table 2'''. System detail of 1 stage 1 elements RO system | ||
[[File:Appendix_7_T2.png|center]] | [[File:Appendix_7_T2.png|center]] | ||
Table 3. System detail of 2 stage 5,5 elements system | '''Table 3'''. System detail of 2 stage 5,5 elements system | ||
[[File:Appendix_7_T3.png|center]] | [[File:Appendix_7_T3.png|center]] | ||
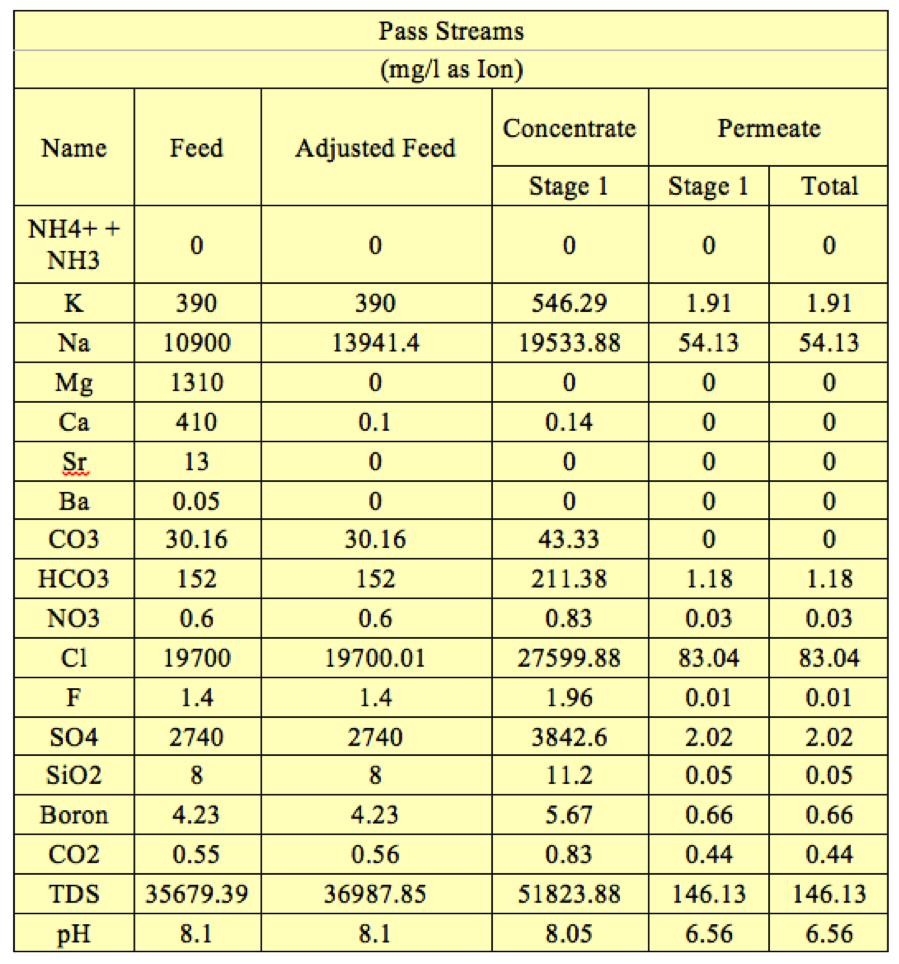
Table 4. 1 stage 5 elements RO system concentrations and pH in each stream | '''Table 4'''. 1 stage 5 elements RO system concentrations and pH in each stream | ||
[[File:Appendix_7_T4.png|center]] | [[File:Appendix_7_T4.png|center]] | ||
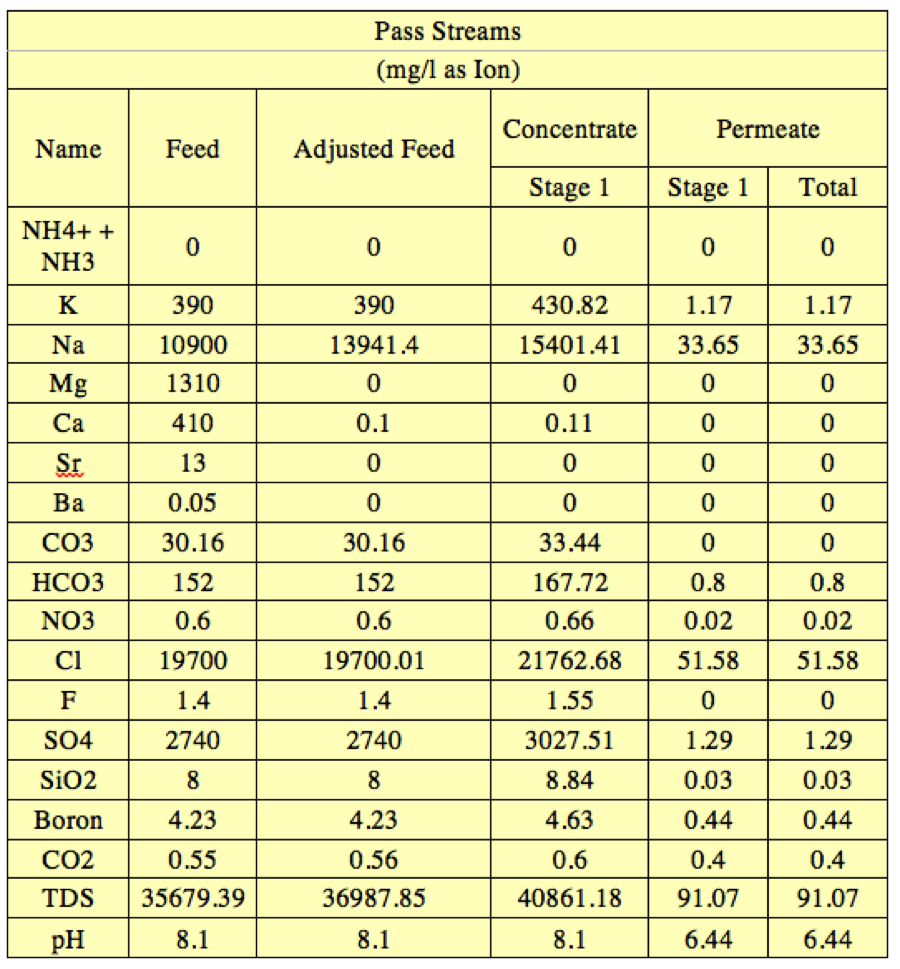
Table 5. 1 stage 1 element RO system concentration and pH in each stream | '''Table 5'''. 1 stage 1 element RO system concentration and pH in each stream | ||
[[File:Appendix_7_T5.png|center]] | [[File:Appendix_7_T5.png|center]] | ||
Table 6. 2 stage 5,5 elements RO system concentrations and pH in each stream | '''Table 6'''. 2 stage 5,5 elements RO system concentrations and pH in each stream | ||
[[File:Appendix_7_T6.png|center]] | [[File:Appendix_7_T6.png|center]] | ||
=References= | =References= | ||
<references/> | <references/> | ||
Revision as of 18:27, 11 March 2016
Group E Corporation
Authors: Hassan Ali, Woo Soo Choe, Brett Sleyster, Jake Stolley
Instructors: Fengqi You, David Wegerer
March 11, 2016
Executive Summary
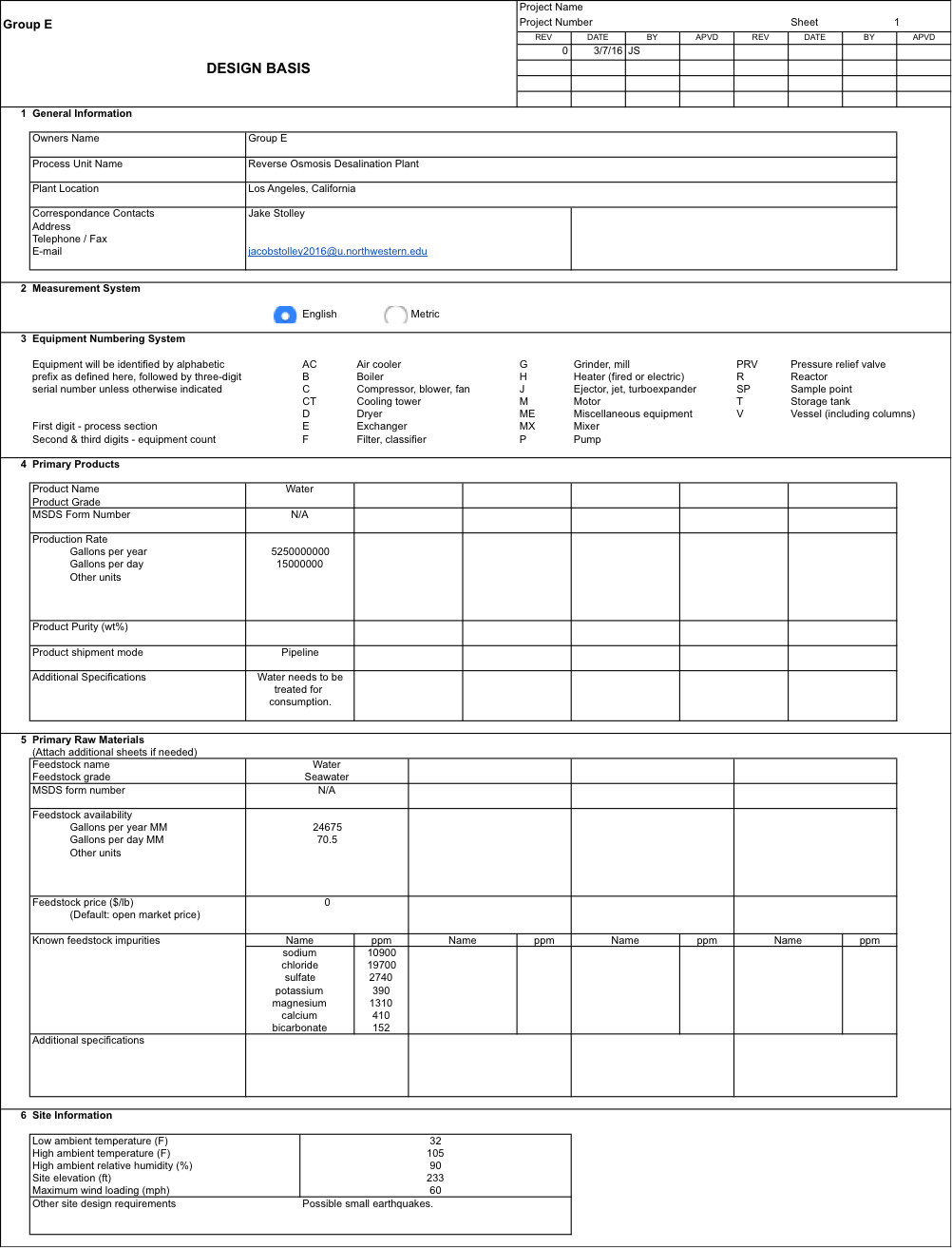
Outlined in this report is the project model report for the proposed desalination plant in Los Angeles, California which will provide drinking water to local citizens using reverse osmosis process. This location was chosen due to the region’s propensity for droughts in recent years. The fresh water resources in California have plummeted as water prices have risen 3-4% each year. This is only expected to continue. Other companies have worked deals with the Californian government to create desalination plants that provide a new fresh water drinking source. This suggests that there is a strong market here with potential growth.
The plant will have an output of 15 million gallons per day, which is about medium size relative to other plants. Thus the build time will be about one year. The plant will run for 30 years, with 350 days per year of use. Shutdown time will be used for cleaning and maintenance of equipment. The plant will be built on Long Beach, 500 meters from the coast nearby the Long Beach Water Reclamation Plant (LBWP). The input will withdraw 70.5 million gallons per day of seawater that will be used for reverse osmosis and dilution of brine.
Reverse osmosis was chosen as the desalination method for its low energy cost and flexibility. We designed a process that will pretreat the seawater to remove waste, colloids, bacteria, and membrane-damaging scalants. Once this seawater is treated is pumped to heavy pressures through a reverse osmosis system. After doing process optimization under various system parameter changes, we found a continuous, single stage, five element reverse osmosis process to be best. It produces a near 30% recovery. We reduce energy costs by including a pressure exchanger between our concentrate outlet and adjusted feed into the reverse osmosis system.
After performing mass and energy balances, we were able to size equipment and find our permeate composition. It turns out our process will meet L.A. standards for drinking water quality. However, post treatment will be done by the local LBWP to make the water taste better and be non-caustic to pipes. This will reduce “in-house” costs. Many environmental considerations were made, to meet our environmental standards. We meet EPA salinity requirements for our brine waste by diluting it. We also don’t use a lot of energy and thus reduce our fossil fuel consumption and CO2 generation. To be safe, we work well under max pressure limits for our equipment. We make sure we are ethical and meet all standards that our local county requires of us.
After an economic evaluation at a conservative well-water price of $700 per acre-foot, we realize that our revenue is way too low to cover our costs. Capital costs are especially large with regards to our large water tanks. We find that we reach a 10 year NPV breakpoint of $2104 per acre-foot after performing a sensitivity analysis. The Carlsbad Plant in San Diego worked out a deal for $2257 per acre-foot.
As we have met all design constraints for this project, we hope to convince policymakers to adopt our project at least $2104 per acre-foot water. If this deal is reached, we recommend that this project and design be funded. There is future work to be done to improve costs such as performing pump optimization. However, this work is minimal and won’t harm our bottom line if we reach our proposed price points.
Introduction
Due to a combination of various factors--including rising human population, climate change, increased energy consumption, and land erosion--the availability of fresh water as a resource for human consumption has been dwindling. This has had a negative impact on the world, but this impact has been especially felt in areas with natural shortages in fresh water. Desert regions, such as Saudi Arabia, have resorted to desalinating seawater at the coast and transporting up to their cities. This encompasses their entire freshwater system as very few natural water sources remain. In the United States there is a region which has shown increasing signs of following in the footsteps of Saudi Arabia and becoming fully reliant on desalination: Los Angeles, California.
Location and Market Analysis
In this project, Los Angeles was chosen as the location our plant, because the area has been subject to a severe drought for the last four years, as shown in Figure 1. The rapid decrease in the available water has caused permanent damages to the ground, decreased the amount of water in the reservoir from 45% to 25%, and reduced the amount of hydroelectric power produced in the state. Such deficiency in usable water has increased the water prices in Los Angeles by 6-7% annually for the last decade and is expected to rise by 3.4% annually for typical customers for the next five years. For customers who use more than 20000 gallons a year, the bill is expected to increase by 34% by 2021. This price increase is occurring because of both drought and maintenance. [1]
As of March 2015 the Metropolitan Water District of Southern California was willing to pay as much as $700 per acre-foot of water to farmers in the Sacramento Valley, so we assume this will be the target price of the water produced by the this desalination plant.[2]
Currently, there is a plant in Carlsbad which supplies drinking water to the San Diego area. The plant can produce up to 50 million gallons per day and is the nation's largest water desalination plant. The water from this plant costs $2257 per acre-foot for the first 48000 acre-feet and about $2000 for any additional acre-foot. The county signed a contract with this plant to purchase the first 48000 acre-feet of water for the next 30 years.[3] Also, there is another desalination plant in Tampa that produces about 25 million gallons of water per day, and over 2000 other public and industrial plants with production capacity of over 300000 gallons per day. When conducting the economic analysis our plant, such numbers presented by other plants must be considered, so our project can yield positive profit.[4]
The profitability of the project is naturally heavily dependent on the price of water in California. If the drought ends, water supply will increase and the water price will drop significantly, but if the drought continues, the price of water in California could rise even more rapidly and allow desalination plants to be more profitable.[5] It may be difficult to make this plant profitable without a guaranteed purchase or other subsidy from the local governments because the presence of pre-existing plants maintains the water price relatively low. However, external sources of profit such as guaranteed purchase or subsidy are expected because the ramifications of not having desalination plant or other sources of water may be significantly more expensive than the subsidizing a desalination plant assuming the drought continues.[6]
Design Basis and Technical Approach
Considering the number of pre-existing desalination plant and the potential risk of the drought ending, our plant will be tentatively designed to produce 15 million gallons of drinkable water per day to homes and businesses in the Los Angeles county area. With a recovery rate of near 30%, and a requirement for diluting any brine, the input feed flowrate will be 70.5 million gallons per day. Following the similarly built Carlsbad plant, we expect the plant to last 30 years. The project building timeline is one year based on our relatively small plant size. We will run for 350 days a year while shutting down for cleaning and maintenance once every 3 months (based on DOW standards).[7] The plant will be built 500 meters from the coastline on Long Beach. A borehole will be drilled 10 meters into the ground. At this depth there should be seawater aquifers below the coast that regularly recharges itself with seawater from the ocean. A vertical well containing intake holes will be put into the borehole. The intake holes will be equipped with large size filters that can easily be replaced. At the surface, the well will be connected to a large-scale pump in a well pump house. From there, the seawater will be horizontally pumped into a seawater tank in the desalination plant. This will be considered the start of our process. The process ends after pretreatment, desalination, post treatment, and brine treatment are completed. Appendix 1 shows more details on the Design Basis.
The feed composition is assumed to be the standard seawater “average” based on the DOW Chemical ROSA 9.0 Technical Manual in Table 1. However, in reality the seawater composition at the plant site will have to be chemically tested. The final product will be based on L.A. standards for drinking water quality (Table 1). The specifications in the table are upper limits that we cannot exceed. We will aim to reach the salt level standards but any remineralization and pH treatment will be exported and paid for due to the added complexity and cost in making such a system in-house.
Due to the acidity of the desalinated water coming out of the reverse osmosis (RO) system (pH ~ 6), the pumps and pipes carrying the desalinated water for post treatment will likely corrode over time. We have decided to place the desalination plant by the Long Beach Water Reclamation Plant (about 50 meters away) to prevent pipe corrosion.
Desalination Overview and Alternatives
We first began our research into the current landscape of the desalination industry, particularly of plants that were built in the United States. We discovered there were many processes but the main ones included:
- Membrane Separation (Forward Osmosis and Reverse Osmosis)
- Thermal Separation (Multi Stage Flash Distillation)
- Phase Change Separation (Freeze Separation)
Each process was analyzed for their pros and cons. For our project design, we decided to go with reverse osmosis as our desalination process. This is because it is the most commonly used process in the industry, and for good reason. Unlike thermal separation, which requires huge amounts of energy to supply heat, reverse osmosis is very energy efficient. This reduces energy costs that would be spread across the plant’s lifetime.[8]
Forward osmosis is a newer technology being developed that also uses membranes to separate water and salt and may be more energy efficient overall than reverse osmosis. This is because forward osmosis doesn’t require a pump to pressurize seawater through the membranes, but rather leaves a higher salt concentrated solution (AKA draw solution) on the other side of the membrane for the seawater to be drawn into. Then a second step is required to regenerate the concentrated draw solution and produce purified water.[9] We decided to move away from forward osmosis because it is a process that is still being refined and developed for desalination and lacks the data, programs, and technologies that reverse osmosis users benefit from. Additionally, a recent study from MIT has claimed that due to the inherent nature of forward osmosis requiring that seawater be drawn to a higher concentrated salt solution before the water and salt is separated, it cannot possibly be more energy efficient than reverse osmosis.[10]
Other processes such as freeze separation were too restrictive in their feed and process demands and required a lot of capital. Ultimately, reverse osmosis is simply the best process that exists for desalination and has been ranked the best when performance is evaluated in terms of technical, environmental, and economic aspects. It is a process that is flexible in water quantity, operation start-up and shut-off, and site location.
Nevertheless there are disadvantages to reverse osmosis that must be considered. The feed seawater must not be too salty and too low quality. The pressure to pump the seawater is enormous. The construction time is long for large plants. The capital costs are also high when compared with other processes such as thermal separation. But all of these disadvantages are ultimately outweighed by the advantages reverse osmosis provides.[8]
Process Overview and Alternatives
See Appendix 2 (Figure 1) for the Process Flow Diagram (PFD). The process itself is detailed below. The stream flow rates are in Appendix 2 (Table 1). We assumed 10 meters of pipe for every stream except the inlet and outlet streams of the entire PFD.
Pretreatment
After the 10 meter vertical well pumps seawater from the Long Beach coast, the feed is pumped 500 meters to a seawater tank that makes it easy to shut-down the system and reduce the large volume of flow. The level control keeps the seawater from overfilling the tank. From there, part of the seawater goes to brine treatment while the rest go to pretreatment. The purpose of pretreatment in the desalination process is to prevent equipment damage by removing particles and microorganisms from the water. A variety of pollutants -- including calcium carbonate, sulfates, silica, clay, bacteria, and other colloids and microorganisms -- can disrupt the reverse osmosis process. Media filtration uses fine grains such as sand or anthracite to filter the particles. We used a large filter to catch larger particle material (e.g. sand). However, simple filtration is not enough to prevent colloid fouling. An ultrafilter (i.e. microfiltration) is used to remove virtually all suspended matter, effectively preventing colloid fouling. The costs associated with ultrafiltration are not prohibitive in terms of the project’s budget.[11]
Reverse osmosis systems can also be disrupted by the growth of microbiological films of bacteria on the film and pipe walls. They are typically treated as colloids during pretreatment, and most organisms are removed from the water by ultrafiltration, but this does not remove the smallest of organic particles. DBNPA is the most effective biocide, but these aren’t often used and have to be removed from the water. Chlorination is an effective way to remove bacteria, but it can cause degradation of the membrane, and must be removed before the reverse osmosis occurs. In our process we will use UV irradiation to remove any organic material that passes through the microfilters.[11]
Scaling can reduce the flux across the membrane and can build up in pipe corners, causing backup. It is caused by inorganic salts -- specifically sulfates and calcium and magnesium carbonate -- present in the water. Scaling can be prevented and/or controlled with acids, antiscalants, or ion exchange softening. Antiscalants are good for removing sulfates, but not much else. Acidification will only be effective at removing CaCO3 from water but, the decreased pH may deteriorate the membrane and pipes, so it may require future research.[11] Ion exchange is very expensive and requires more capital to undergo, but is overall the more effective option.
The ion exchanger is a pretreatment system which acts as a method to fight membrane scaling in reverse osmosis. The ion exchanger works by using sodium carboxylate as a resin which will react with Chlorine and Magnesium ions, removing them from the feed. The sodium is substituted out and enters the feed stream, increasing the overall sodium level and TDS. The lack of magnesium and chlorine ions means that there is nothing to react with the carbonates to form scaling salts on the membranes.
Desalination (Reverse Osmosis)
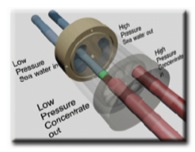
Two pumps (P-105 & P-106) take the adjusted feed seawater up to 58 bar. However, before this pumping occurs, about 70% of the original stream gets separated and heads into a PX Pressure Exchanger. This separated stream later joins together with the pumped stream to enter the reverse osmosis system at the required pressure of 58 bar. The streams can combine together as they have equal pressure. It is the concentrate stream from the output of the reverse osmosis system that transfers its energy to the adjusted feed in the PX Pressure Exchanger.
The pressure of the concentrate stream is about 57 bar and it is fed into an exchanger which works by using vessel that resembles a small pipe within a rotating wheel. The low pressure (LP) feed enters the vessel at 1 bar (see Figure 3). The feed turns and is pushed out into an outlet pipe by the high pressure (HP) concentrate stream at ~57 bar. The feed is now a HP stream via this exchanged energy. The now LP concentrate stream turns and is pushed out into an outlet pipe by the LP feed entering the vessel.
98% of the energy within the concentrate stream can be recovered via the PX Pressure Exchanger.[12]Thus, the now HP feed stream is not quite 58 bar and will need to be pumped by a small booster pump (P-107).
When designing a reverse osmosis system, we need to make decisions on what specific kind of reverse osmosis system will be implemented. When making the decision, we investigated the advantages of different types of reverse osmosis among the following options: batch vs. continuous process, number of stages, and number of elements.[13] Our decision was made based on process optimization which is described later in this report. The end result of that optimization was to go with a single stage, continuous process with five elements in series.
Post Treatment
The permeate is sent to post treatment after the salt content is analyzed and controlled if too high or too low due to any unexpected errors. As stated above, the post treatment process will be done 50 meters from our plant by the Long Beach Water Reclamation Plant. The reason we aren’t performing post treatment “in-house” is due to the increased cost it would take for us to build and run the equipment for post treatment. We have the option to use the government’s water treatment facilities that already exist and our 15 million gallons per day output is a relatively small addition to the large Long Beach Water Reclamation Plant. We will assume any costs to post treatment will be pushed to the consumer in the price of purchasing the drinking water from the Long Beach Water Reclamation Plant.
Post treatment at any facility works to bring water to “taste standards”. The water that is the permeate of our process is already meeting L.A. drinking standards as shown above in Table 1 after our mass balance calculations around the reverse osmosis system (Appendix 3, Table 1). The post treatment increases the pH from 6.3 to 7.3 with the addition of base and then adds minerals like fluoride, calcium, and magnesium back in to improve taste.
Brine Treatment and Byproduct Waste
A natural consequence of a reverse osmosis procedure is that a lot of salt will be leftover from the membrane separation in a very concentrated form. This is called “brine” and will have to be taken care of. According to EPA requirements, the salt concentration of water entering the environment (via land or sea) cannot be higher than 40,000 ppm.8 The salt concentration of our brine is much higher (around 70,000 ppm). Thus, the brine must be removed or treated. We decided to treat the brine by diluting it as removing brine can be difficult and may require lots of land (e.g. evaporation ponds).
After the concentrate exchanges energy in the energy exchanger, it becomes a low pressure stream. It is pumped into a dilution tank and mixes with 18.2 million gallons per day of seawater from the inlet to become diluted brine which is pumped 600 meters out to sea.
The waste byproducts are the solid buildup on the filters and membranes. The filters and membranes also have a set lifetime and thus will also need to be removed. The waste is all non-hazardous and thus can be put in the municipal dump.
Process Details and Equipment Sizing
Filters and UV Treatment [Pretreatment]
The cost of our ultrafiltration system is estimated using data from the University of Tennessee at Knoxville.[14] It estimated the cost of the cost of the ultrafiltration $0.057 per 1000 gallons and the construction and capital costs are $0.39 per gallon per day. The cost of UV treatment is based off of units available from buyultraviolet.com for units that can treat about 450 gallons per minute.[15] Due to the water having to be in relatively small pipes for treatment we do not expect the costs to scale very much, so we believe these units to be an accurate cost estimate.
Ion Exchanger [Pretreatment]
The ion exchanger was sized using data from DOW. Our process will use 60 vessels with ion exchange beds of volume 5.73 m3. With a flow rate of 30 bed volumes/hour (10,312.2 m3/hr) the system of beds has a capacity of 125% of the feed. This allows for periodic regeneration of the resin, a weak acid cation resin (DOW MAC-3 Resin). This weak cation resin will remove cationic calcium and magnesium from the water to prevent scaling. The mass flow was calculated via a mass balance on the ion exchanger (Appendix 3).
Reverse Osmosis System [Desalination]
Each of the 2607 Reverse Osmosis SW30HRLE-440i [Seawater 30 High Rejection Low Energy 440 ft2 interlocking cap membrane] units have the same dimensions. A, the net length, is 1016 mm. B, the total length including the endcap, is 1029 mm. C, the diameter of the filter element, is 201mm. D, the diameter of the permeate pathway, is 29 mm. For a single stage, we have five of the elements in series so the overall length of a single stage is expected to be roughly 5145 mm. The membrane material is polyamide thin film composite. It will last three years.
Operating Conditions [ROSA 9.0]:
- Temperature: 30oC
- Pressure: 58 bar
- Flow Rate: 52.3 Million gal per day
- Recovery: 28.7%
Cleaning Schedule:
- Conservative Estimate: Once every three months (three days available for cleaning)[7]
- DOW FILMTEC Cleaning Conditions
- The normalized permeate flow drops 10%
- The normalized salt passage increases 5 - 10%
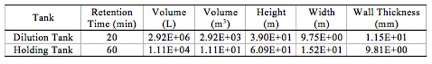
Seawater Holding Tank and Dilution Tank [Brine Treatment]
The initial seawater holding tank works as a buffer to prevent surges and help perform shutdowns quickly and easily. We expect to run continuously for 350 days a year with the rest of the available time spread out and used for shutting down the plant for cleaning membranes, filters, and the fixing of unexpected errors. There is a big tradeoff between having a holding tank with high retention time and having a holding tank with low cost. With that in mind, we erred on the side of lowering capital cost and went with a retention time of one hour. The volume could be found by multiplying the retention time with the feed flow rate (Stream 1). We assumed that a good height to width ratio for a large water tank would be 4:1.
A mass balance was done around the dilution tank to find what amount of seawater would be necessary to dilute the brine to 45,000 ppm salinity (Appendix 3; Table 3). With this the feed flow rate into the tank could be found. It was found that it takes about 20 minutes of retention time for good dilution of the brine in seawater.8 We again assumed that a good height to width ratio for a large water tank would be 4:1.
We assumed a corrosion allowance of 4 mm wall thickness. We calculated wall thickness using vessel pressure, hoop stress, longitudinal stress, and weld factor. Each tank is made with SS316 to protect against corrosion.[16]
Table 2. Tank sizes.
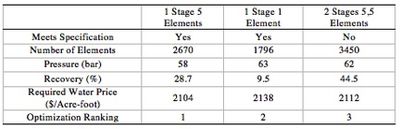
Process Optimization
In order to optimize the reverse osmosis system, numerous simulations were run. Three representative cases are reported as shown above (Table 3). The objective of the optimization was to minimize the required water price to break even in 10 years. In order to achieve this goal, the balance between the capital cost from the number of the filter elements and utility cost from energy consumption was weighed. Before the energy recovery system was implemented, the general trend was that if we have more filter elements in series, overall recovery increases but recovery rate per filter decreases, and we need more filter elements. While increasing the elements in series increase the required number of filters, and thereby increases the capital cost, we require less energy to produce the same amount of water. After the energy recovery system is implemented, the correlation between the number of elements in series and the required energy becomes more complicated because high pressure concentrate is used to pressurize the feed. However, the trade-off between the utility cost and capital cost still exists. Of the three cases shown above, single stage, five elements RO system turned out to be the most optimal solution because single stage, single element system required higher utility cost and two stage system slightly did not meet the LA standard. Detailed information which was used for the optimization is available in Appendix 3 and in Appendix 6.
Ethical, Environmental, and Societal Considerations
There are a few key considerations we have followed to meet ethical, environmental, and societal guidelines and requirements:
- Meet L.A. Drinking Water Standards
- Correct ion concentration
- Moved near water treatment plant
- Pipes will not corrode from low pH
- Working below pressure limits
- Water will be locally served and meet taste standards
- Via Post Treatment mineralization
- Follows EPA Environmental Salinity Requirements
- Reduced Energy Requirements
- PX Pressure Exchanger
- Optimized RO System
- Reverse Osmosis is itself Energy Saving
For further environmental considerations, see effluent summary in Appendix 4.
Economic Analysis
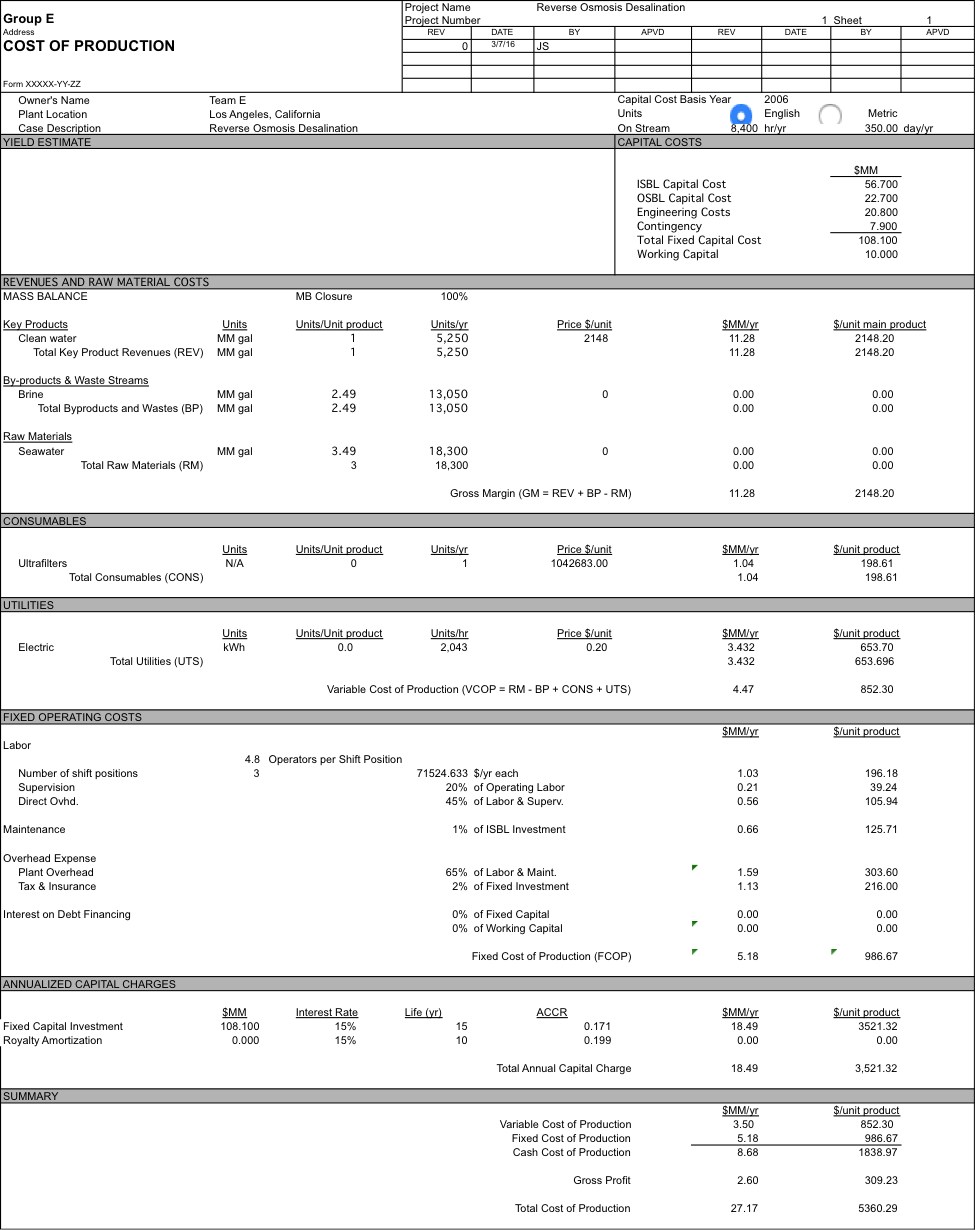
The pricing of the majority of the process was completed using the Aspen Economic Analyzer, however we had determine costs for the ultrafiltration, reverse osmosis membranes, and the ultraviolet treatment was completed separately. This led to a total ISBL of $56 MM, OSBL of $22.4 MM, Engineering Costs of $22.4 MM and a Contingency of $7.8 MM. We also chose a working Capital of $10 MM. This working capital is sufficient because we have no raw materials costs. We also had FCOP of $5.2 MM and VCOP of $3.5 MM.
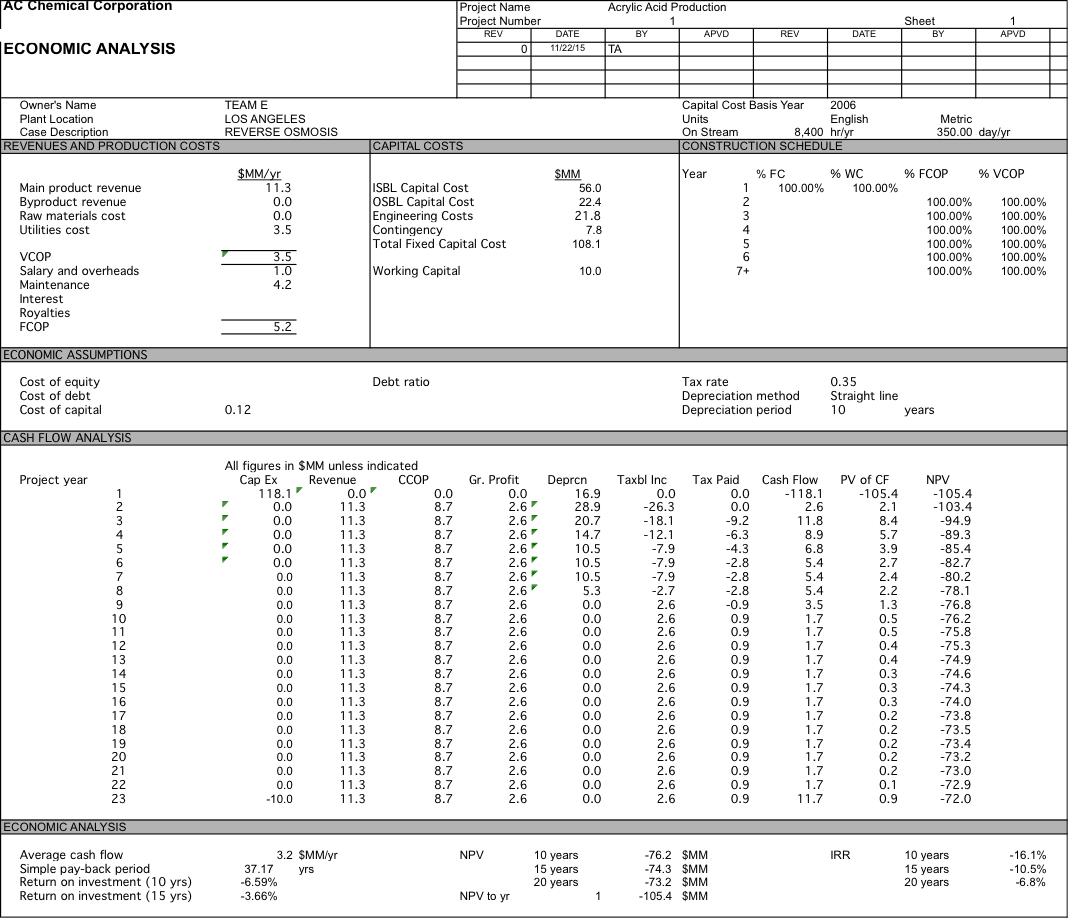
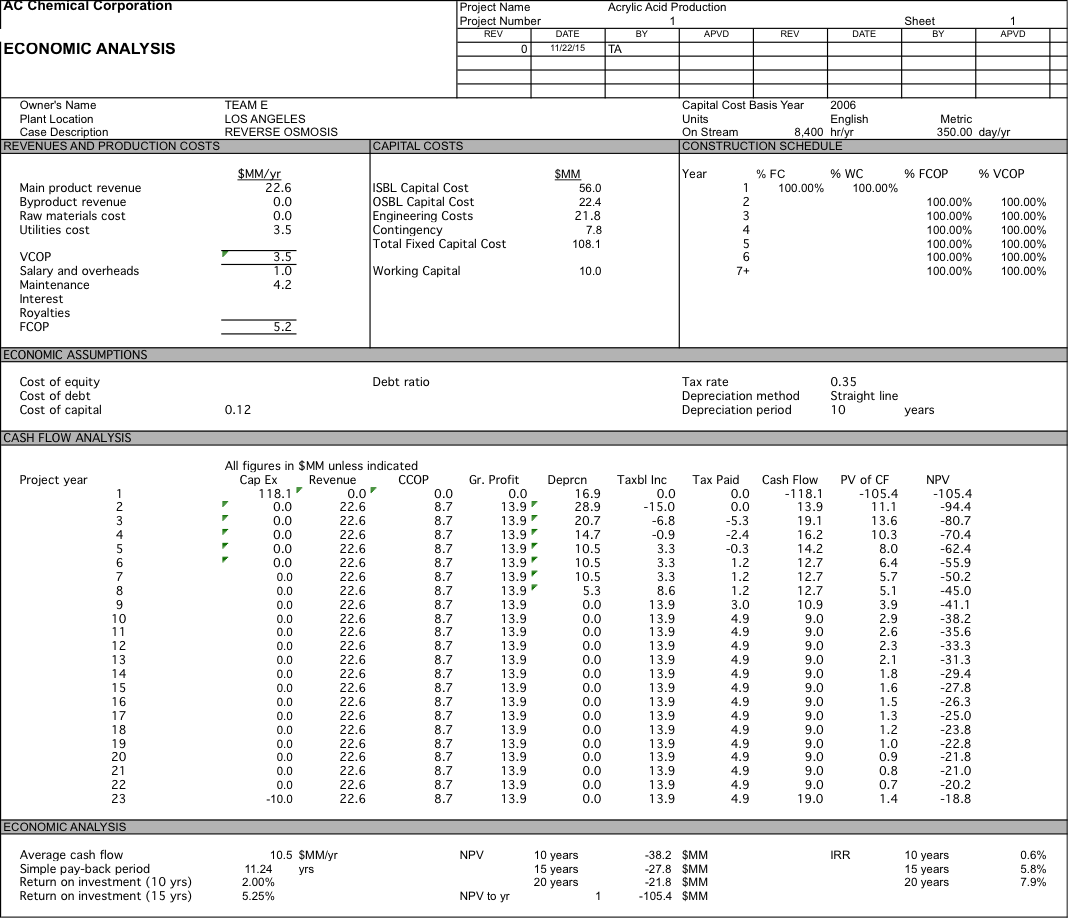
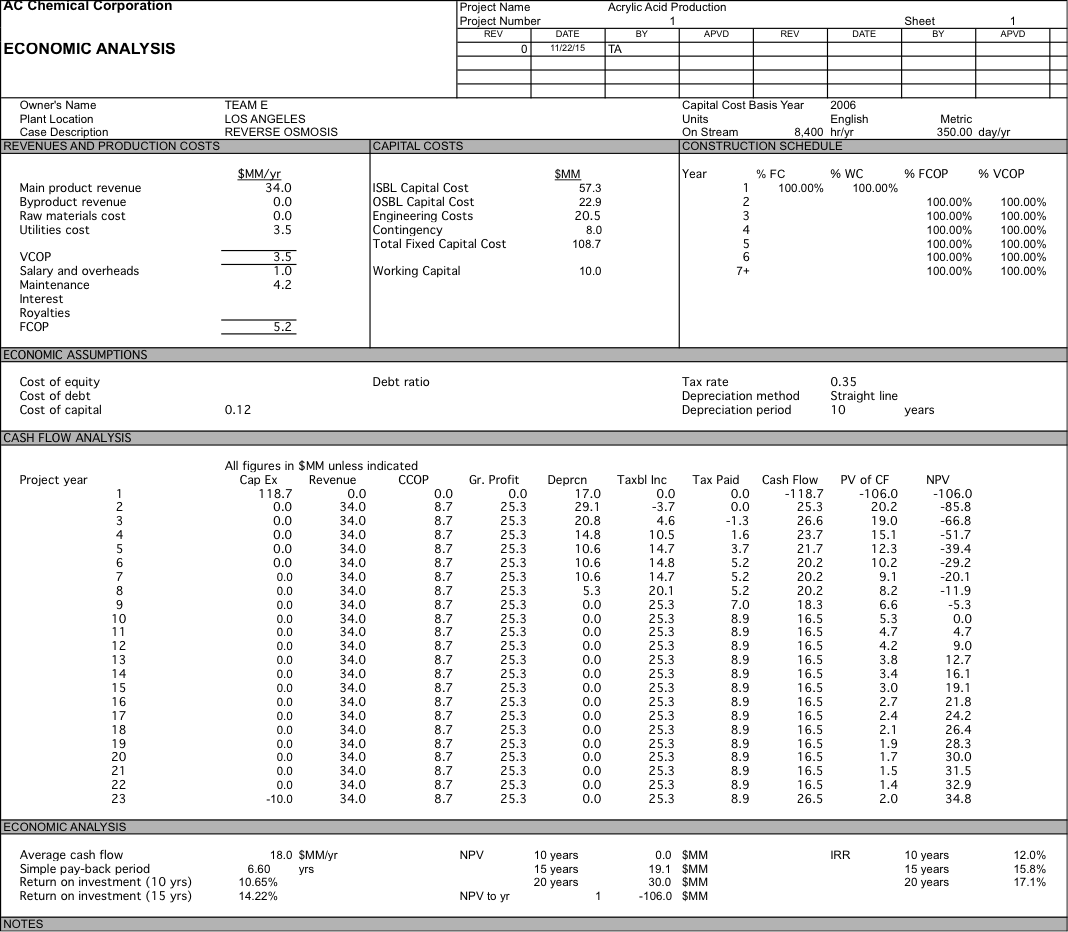
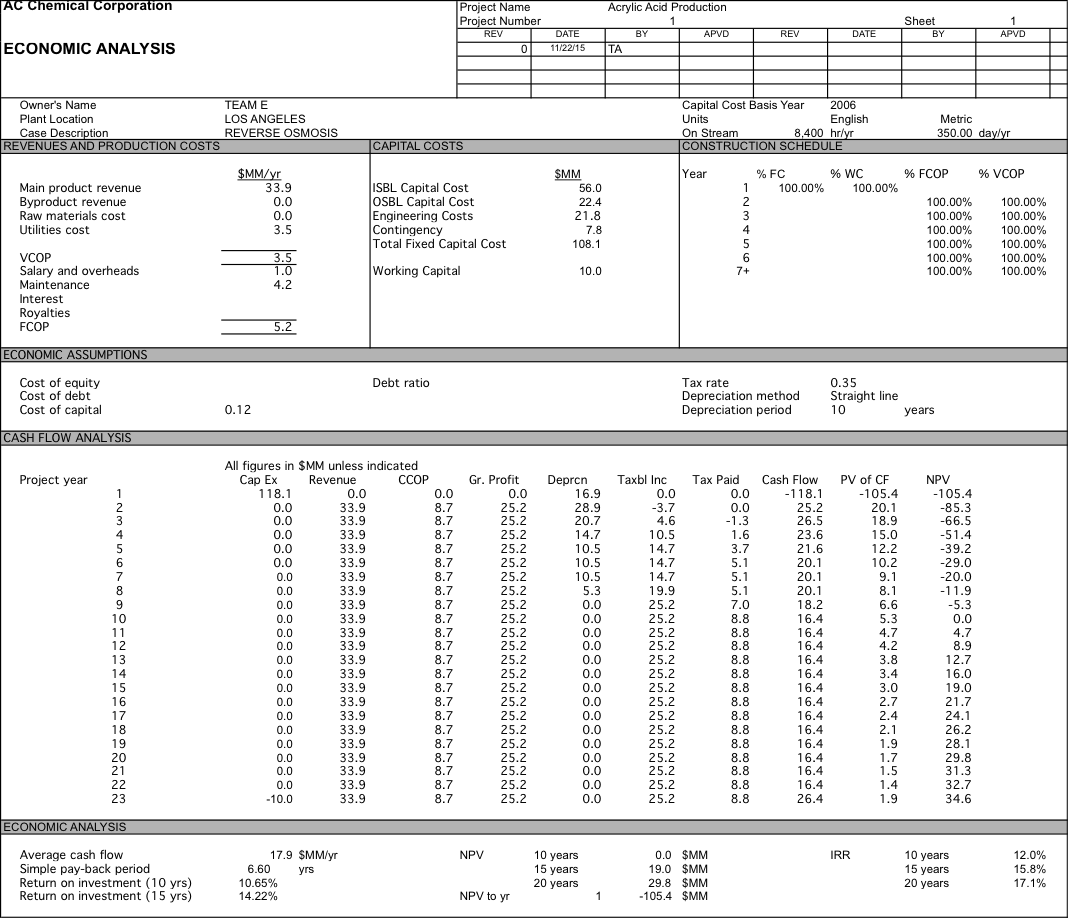
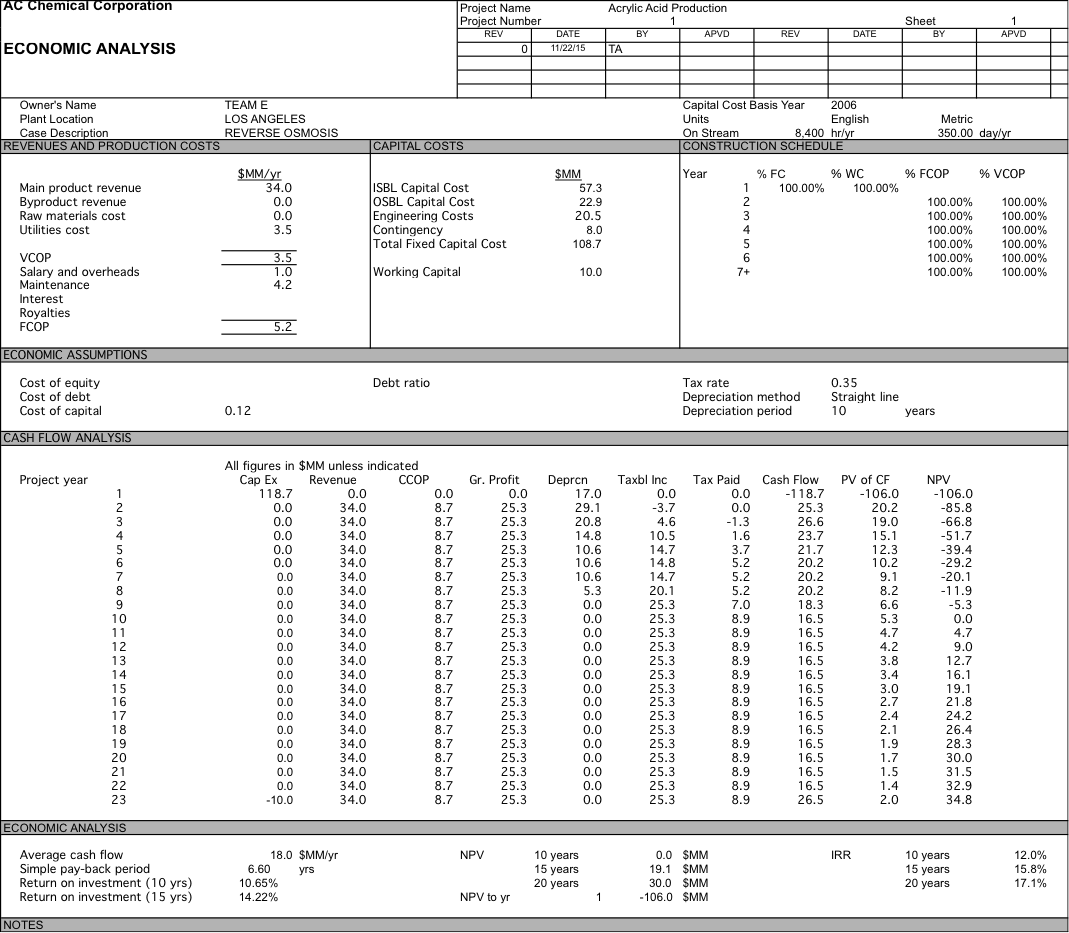
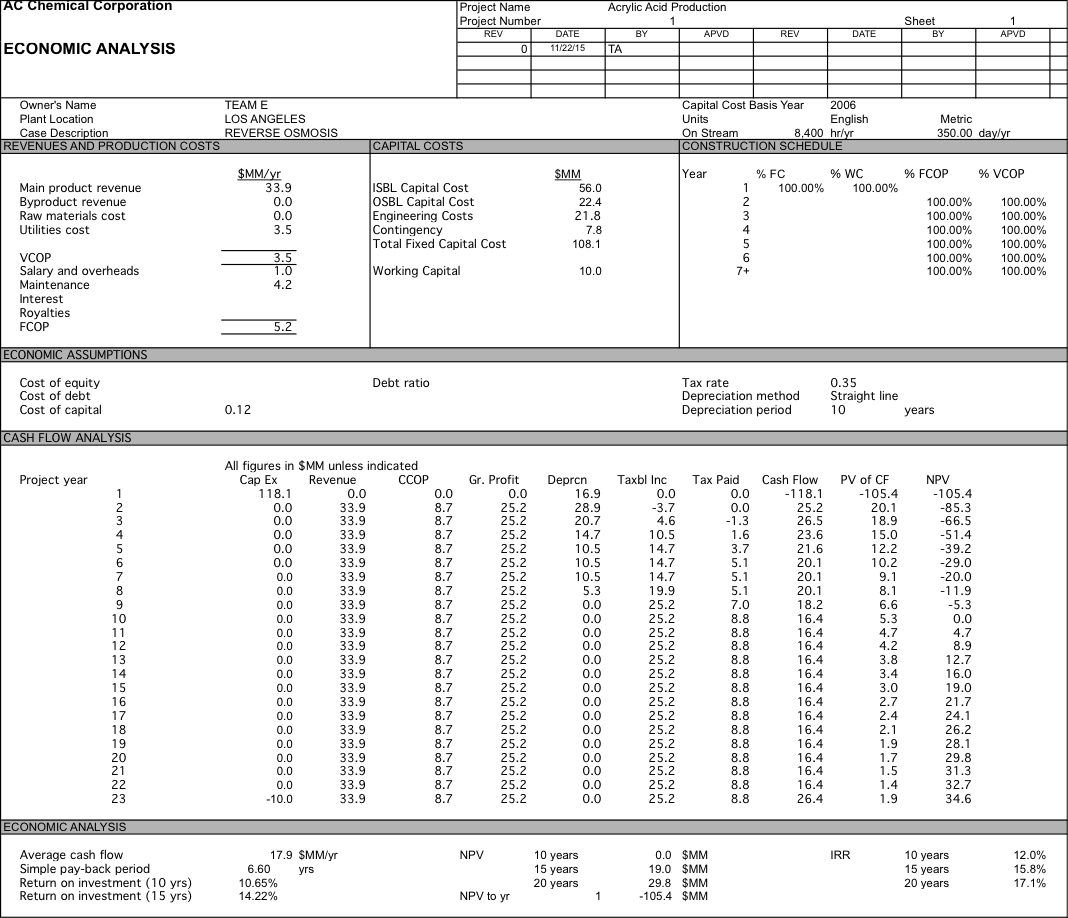
We originally were planning on pricing our water at $700 per acre-foot, but after doing economic analysis we had a 10 year NPV of $-76.2 MM. After determining did a sensitivity analysis and determined that if our water was priced at $2104 per acre-foot we could have an NPV of $0 after 10 years and if we were able to get the same price of $2257 per acre-foot as the plant in Carlsbad we would have a 10 year NPV of $8.3 MM which increases to $41.1 MM at 20 years. For more detailed data about the economic analysis see Appendix 6.
Conclusion and Recommendations
Our mission was to create an economically viable process that will desalinate water for human consumption in the L.A. County. We have found after research and optimization that the best process to undergo desalination is a continuous single stage, five elements reverse osmosis system. Our process can meet the required L.A. drinking water standard and we have set up plans for post treatment at a local water reclamation site. While meeting the drinking water standards, we also met our ethical, environmental, and social standards.
Nevertheless, at a sale price of $700 per acre-foot, our proposed desalination plant in Los Angeles, California looks to be a very expensive project to undergo. Without any sort of government assistance to carry the heavy capital costs of such a project, we would suggest the plant not be built. However, after performing a sensitivity analysis at higher (and less conservative) price points we reached a breakeven point in 10 years with a sale price of $2104 per acre-foot of drinking water. Research has shown that the droughts striking California look only to get worse in the near future. With limited freshwater resources, there is no option other than to consider desalination. Therefore it is reasonable to expect we could get near the same price deal as local desalination plants like the Carlsbad Plant ($2257 per acre-foot) As we have met all design constraints for this project, we hope to convince policymakers to adopt our project at least $2104 per acre-foot water. If this deal is reached, we recommend that this project and design be funded.
However, there are ways to improve our design with future work. Right now all the pipes diameters are based on having a flowrate of 1.5 m/s. But in reality, we’d need to use standard piping sizes to get more accurate pricing and energy costs. Also, in reality we would be purchasing some of these vessels in bulk quantities (i.e. bulk filters) so the price could be lowered with negotiation and economies of scale. We also have pumps that are providing very little pressure for their high cost. We can remove these pumps and simply run at higher pressures earlier. We can potentially reduce early energy costs by building a “floating” desalination plant in the sea to reduce pumping distance from the well.
Appendices
Appendix 1: Design Basis
Appendix 2: Process Flow Diagram
Appendix 3: Mass and Energy Balance Tables
Table 1. Mass balance on 1 stage 5 element RO system, where "out" is the sum of all the concentrate and permeate.
Table 2. Mass balance on 1 stage 1 element RO system, where "out" is the sum of all the concentrate and permeate.
Table 3. Mass balance on 2 stage 5,5 element RO system, where "out" is the sum of all the concentrate and permeate.
Table 4. 1 stage 5 elements CO3, HCO3, and CO2 ion mass balance
Table 5. 1 stage 1 element CO3, HCO3, and CO2 ion mass balance
Table 6. 2 stages 5,5 elements CO3, HCO3, and CO2 ion mass balance
Table 7. Energy balance on 1 stage 5 elements RO system
Appendix 4: Effluent Summary
Appendix 5: Cost of Production
Appendix 6: Economic Anlaysis
Case I
Water is $700; RO with 1 stage and 5 elements
Case II
Water is $1400; RO with 1 stage and 5 elements
Case III: Carlsbad
Water is $2257; RO with 1 stage and 5 elements
Case IV: Breakeven (NPV = 0 at 10 years)
Water is $2104; RO with 1 stage and 5 elements
Case V: Breakeven (NPV = 0 at 10 years)
Water is $2112; RO with 2 stages and 5,5 elements
Case VI: Breakeven (NPV = 0 at 10 years)
Water is $2138 with 1 stage and 1 element
Appendix 7: ROSA Simulation Outputs
Table 1. System detail of 1 stage 5 elements RO system
Table 2. System detail of 1 stage 1 elements RO system
Table 3. System detail of 2 stage 5,5 elements system
Table 4. 1 stage 5 elements RO system concentrations and pH in each stream
Table 5. 1 stage 1 element RO system concentration and pH in each stream
Table 6. 2 stage 5,5 elements RO system concentrations and pH in each stream
References
- ^ Pacific Institute: The California Drought. 2014.
- ^ Hessel, Phil. “Dry Southern California Offers Northern Farmers Top Dollar for Water”. NBC News. March 18, 2015.
- ^ Carlsbad Desalination Plant. “Project Overview”. January 2016.
- ^ Leven, Rachel. “U.S. Desalination Industry Grows Since 2000; See as Essential to Meeting Supply Needs”. Bloomberg BNA. August 21, 2013.
- ^ Southern California Public Radio. “Where is California water use decreasing?”. December 2015.
- ^ Weiser, Matt. “Could desalination solve California’s water problem?”. The Sacramento Bee. October 18, 2014.
- ^ a b Bates, Wayne. “Cleaning your RO”. Hydranautics.
- ^ a b Republic of Mauritius NY. "Technical Aspects of Desalination Plant". Sustainable Sanitation and Water Management.
- ^ Mallinson, Alissa. "Study Shows Forward Osmosis Desalination Not Energy Efficient." MIT News [Boston]. July 23, 2014.
- ^ O'Hern, Sean C., Karnik, Rohit. Nanofiltration across Defect-Sealed Nanoporous Monolayer Graphene. American Chemical Society 15.5 (2014); 3254-260.
- ^ a b c Desalination Pre-treatment. Lenntech.
- ^ Desalination Product and Applications Catalog 2013. “Advance Technologies for Seawater Reverse Osmosis and Brackish Water Applications”. Energy Recovery, Inc. 2012-2014.
- ^ FILMTEC Reverse Osmosis Membranes. “Water & Process Solutions Technical Manual.” Dow Chemical Company.
- ^ Qiang He and R. Bruce Robinson Membrane Filtration Systems. “Membrane Module Costs.” University of Tennessee at Knoxville. December 2014.
- ^ Atlantic Ultraviolet Corporation. “Megatron Water Disinfection System.”
- ^ Towler G, Sinnott R. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design. 2nd ed. Boston: Elsevier; 2013.